Electrochemical Determination of Epinephrine in Pharmaceutical Preparation Using Laponite Clay-Modified Graphene Inkjet-Printed Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Characterizations of Non-Modified and La-Modified Inkjet-Printed Graphene Electrodes
2.2. EIS Analysis of Bare IPGE and La/IPGE
2.3. Electrochemical Detection of EP on the IPGE Modified Electrode
2.3.1. Electrochemical Behavior of EP by Cyclic Voltammetry
2.3.2. Influence of the Detection Medium
2.3.3. Influence of Scan Rate on the Electrochemical Response of EP on La/IPGE
2.3.4. Influence of pH on the Electrochemical Response of EP
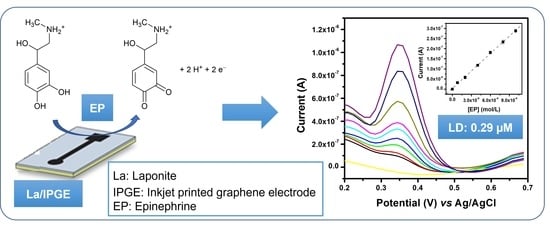
2.3.5. Influence of EP Concentration and Calibration Curve
2.4. Interference Study
2.5. Analytical Application
3. Conclusions
4. Experimental Section
4.1. Chemicals, Reagents and Materials
4.2. Preparation of Inkjet-Printed Graphene Electrode (IPGE)
4.3. Apparatus
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hussain, A.; Truelove, J.E. Prodrug approaches to enhancement of physicochemical properties of drugs IV: Novel epinephrine prodrug. J. Pharm. Sci. 1976, 65, 1510–1512. [Google Scholar] [CrossRef]
- Shoukas, A.A.; Brunner, M.C. Epinephrine and the carotid sinus baroreceptor reflex. Influence on capacitive and resistive properties of the total systemic vascular bed of the dog. Circ. Res. 1980, 47, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.D.; Bonito, A.J.; Shugars, D.A. A systematic review of cardiovascular effects of epinephrine on hypertensive dental patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 647–653. [Google Scholar] [CrossRef]
- Hague, S.M.; Klaffke, S.; Bandmann, O. Neurodegenerative disorders: Parkinson’s disease and Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1058–1063. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Shen, L.; Lu, J. Flow injection chemiluminescence determination of epinephrine using epinephrine-imprinted polymer as recognition material. Anal. Chim. Acta 2003, 489, 183–189. [Google Scholar] [CrossRef]
- Britz-Mckibbin, P.; Wong, J.; Chen, D.D. Analysis of epinephrine from fifteen different dental anesthetic formulations by capillary electrophoresis. J. Chromatogr. A 1999, 853, 535–540. [Google Scholar] [CrossRef]
- Wang, Y.; Fice, D.S.; Yeung, P.K. A simple high-performance liquid chromatography assay for simultaneous determination of plasma norepinephrine, epinephrine, dopamine and 3, 4-dihydroxyphenyl acetic acid. J. Pharm. Biomed. Anal. 1999, 21, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Jesny, S.; Sivasankaran, U.; Kumar, K.G. Fluorometric determination of epinephrine: A green approach. Anal. Sci. 2016, 32, 999–1001. [Google Scholar] [CrossRef] [Green Version]
- Tonle, I.K.; Ngameni, E.; Tchieno, M.M.F.; Walcarius, A. Organoclay-modified electrodes: Preparation, characterization and recent electroanalytical applications. J. Solid State Electrochem. 2015, 19, 1949–1973. [Google Scholar] [CrossRef]
- Mousty, C. Biosensing applications of clay-modified electrodes: A review. Anal. Bioanal. Chem. 2010, 396, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Mattos, G.J.; Mariano, R.S.; Sartori, E.R. Recent advances in electrochemical monitoring of epinephrine using carbon-based (bio) sensor devices for clinical applications. In Electrochemical Sensors Based on Carbon Composite Materials: Fabrication, Properties and Applications; Manjunatha, J.G., Ed.; IOP Publishing: Bristol, UK, 2022; pp. 9–27. [Google Scholar]
- Karim-Nezhad, G.; Khorablou, Z. Selective analysis of epinephrine in the presence of uric acid by using an amplified electrochemical sensor employing a gold nanoparticle decorated cysteic acid film. Anal. Methods 2017, 9, 6394–6402. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, P.; Xie, Z.; Ni, M.; Wang, C.; Yang, P.; Xie, Y.; Fei, J. Selective determination of epinephrine using electrochemical sensor based on ordered mesoporous carbon/nickel oxide nanocomposite. Talanta 2021, 233, 122545. [Google Scholar] [CrossRef]
- Gunes, O.; Sarilmaz, A.; Zeki Bas, S.; Ozmen, M.; Ozel, F.; Ersoz, M. Electrochemical detection of epinephrine based on a screen-printed electrode modified with nio–ergo nanocomposite film. Electroanalysis 2021, 33, 2460–2468. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Reddy, K.R.; Aminabhvi, T.M. Graphene–Clay-based hybrid nanostructures for electrochemical sensors and biosensors. In Micro Nano Technologies, Graphene-Based Electrochemical Sensors for Biomolecules; Pandikumar, A., Rameshkumar, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 10; pp. 235–274. ISBN 9780128153949. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékany, I. Clay Minerals Organic Interactions in Handbook of Clay Science, 2nd ed.; Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 435–505. [Google Scholar]
- Li, X.-L.; Jiang, G.-C.; Xu, Y.; Deng, Z.-Q.; Wang, K. A new environmentally friendly water-based drilling fluids with laponite nanoparticles and polysaccharide/polypeptide derivatives. Petrol. Sci. 2022, 19, 2959–2968. [Google Scholar] [CrossRef]
- Perotti, G.F.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J.L.; Constantino, V.R.L. Bacterial cellulose–laponite clay nanocomposites. Polymer 2011, 52, 157–163. [Google Scholar] [CrossRef]
- Valencia, G.A.; Freitas Moraes, I.C.; Gilles Hilliou, L.H.; Lourenço, R.V.; do Amaral Sobral, P.J. Nanocomposite-forming solutions based on cassava starch and laponite: Viscoelastic and rheological characterization. J. Food Eng. 2015, 166, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.; Zen, J.M.; Kumar, A.S.; Chen, P.Y. Flow injection analysis of zinc pyrithione in hair care products on a cobalt phthalocyanine modifed screen-printed carbon electrode. Talanta 2004, 62, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfac. Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Corona-Avendaño, S.; Alarcón-Angeles, G.; Rojas-Hernández, A.; Romero-Romo, M.A.; Ramírez-Silva, M.T. Study on the stability of adrenaline and on the determination of its acidity constants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Fritsch, I. Application of electrochemical redox cycling: Toward differentiation of dopamine and norepinephrine. Anal. Chem. 2016, 88, 5574–5578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoneim, M.M.; Hassanein, A.M.; Hammam, E.; Beltagi, A.M. Simultaneous determination of Cd, Pb, Cu, Sb, Bi, Se, Zn, Mn, Ni, Co and Fe in water by differential pulse stripping voltammetry at a hanging mercury drop electrode. Fres. J. Anal. Chem. 2000, 367, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Turkušić, E.; Redžić, S.; Kahrović, E.; Zahirović, E. Electrochemical determination of adrenaline at Ru(III) Schiff base complex modified carbon electrodes. Croat. Chem. Acta 2017, 90, 345–352. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Y.; Lin, X.; Tang, Y.; Liu, A.; Li, G.; Li, W.; Zhang, S. Selective determination of epinephrine in the presence of ascorbic acid and uric acid by electrocatalytic oxidation at poly(eriochrome black-t) film-modified glassy carbon electrode. Anal. Sci. 2007, 23, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Babaei, A.; Mirzakhani, S.; Khalilzadeh, B. A sensitive simultaneous determination of epinephrine and tyrosine using an iron(III) doped zeolite-modified carbon paste electrode. J. Braz. Chem. Soc. 2009, 20, 1862–1869. [Google Scholar] [CrossRef]
- Ren, W.; Luo, H.Q.; Li, N.B. Simultaneous voltammetric measurement of ascorbic acid, epinephrine and uric acid at a glassy carbon electrode modified with caffeic acid. Biosens. Bioelectron. 2005, 21, 1086–1092. [Google Scholar] [CrossRef]
- Tajeu, K.Y.; Ebunang, D.V.T.; Tonleu, R.C.T.; Jiokeng, S.L.Z.; Ymele, E.; Tonle, I.K. Electroanalytical application of thiol-grafted laponite to the sensitive quantification of ciprofloxacin antibiotic. J. Appl. Electrochem. 2021, 51, 435–446. [Google Scholar] [CrossRef]
- Tcheumi, H.L.; Tonle, I.K.; Ngameni, E.; Walcarius, A. Electrochemical analysis of methylparathion pesticide by a gemini surfactant-intercalated clay-modified electrode. Talanta 2010, 81, 972–979. [Google Scholar] [CrossRef]
- Guenang, S.L.; Dongmo, M.L.; Jiokeng, Z.L.S.; Kamdem, T.A.; Doungmo, G.; Tonle, I.K.; Bassetto, V.C.; Milica, J.; Lesch, A.; Girault, H. Montmorillonite clay-modified disposable ink-jet-printed grapheme electrode as a sensitive voltammetric sensor for the determination of cadmium(II) and lead(II). SN Appl. Sci. 2020, 2, 476. [Google Scholar] [CrossRef] [Green Version]










| Modified Electrodes | Concentration Range (µM) | Detection Limit (µM) | Supporting Electrolyte (pH) | Methods | Ref. |
|---|---|---|---|---|---|
| GCE/Na[RuL2] | 3–136 | 3.49 | 0.1 M PB (pH 7.5) | FIA | [27] |
| ECS/Na[RuL2]/cellulose acetate | 3–273 | 0.289 | 0.1 M PB (pH 7.5) | FIA | [27] |
| Poly(EBT)-modified GCE | 2.5–50 | 0.3 | 0.1 M PB (pH 3.5) | DPV | [28] |
| Iron(III) doped zeolite-modified CPE | 0.9–216 | 0.44 | 0.1 M PB (pH 3.0) | DPV | [29] |
| Caffeic acid-modified GCE | 2–300 | 0.60 | 0.15 M PB (pH 7.4) | CV | [30] |
| Bare IPGE | 0.8–10 | 0.34 | 0.1 M AB (pH 3.75) | DPV | This work |
| Laponite clay-modified IPGE | 0.8–10 | 0.26 | 0.1 M AB (pH 3.75) | DPV | This work |
| Sample | Dilution Rate | [EP] Added (µM) | [EP] Founded (µM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 100 | 54.58 | 51.45 | 94.26 |
| 2 | 400 | 18.19 | 20.34 | 102.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecheu, C.N.; Tchieda, V.K.; Tajeu, K.Y.; Jiokeng, S.L.Z.; Lesch, A.; Tonle, I.K.; Ngameni, E.; Janiak, C. Electrochemical Determination of Epinephrine in Pharmaceutical Preparation Using Laponite Clay-Modified Graphene Inkjet-Printed Electrode. Molecules 2023, 28, 5487. https://doi.org/10.3390/molecules28145487
Pecheu CN, Tchieda VK, Tajeu KY, Jiokeng SLZ, Lesch A, Tonle IK, Ngameni E, Janiak C. Electrochemical Determination of Epinephrine in Pharmaceutical Preparation Using Laponite Clay-Modified Graphene Inkjet-Printed Electrode. Molecules. 2023; 28(14):5487. https://doi.org/10.3390/molecules28145487
Chicago/Turabian StylePecheu, Chancellin Nkepdep, Victor Kougoum Tchieda, Kevin Yemele Tajeu, Sherman Lesly Zambou Jiokeng, Andreas Lesch, Ignas Kenfack Tonle, Emmanuel Ngameni, and Christoph Janiak. 2023. "Electrochemical Determination of Epinephrine in Pharmaceutical Preparation Using Laponite Clay-Modified Graphene Inkjet-Printed Electrode" Molecules 28, no. 14: 5487. https://doi.org/10.3390/molecules28145487