8(meso)-Pyridyl-BODIPYs: Effects of 2,6-Substitution with Electron-Withdrawing Nitro, Chloro, and Methoxycarbonyl Groups
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
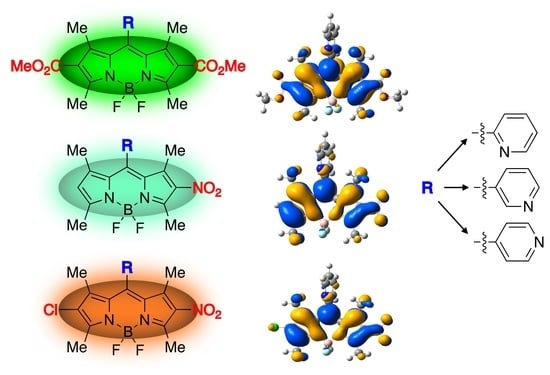
2.2. X-ray and Computational Structural Analysis
2.3. Spectroscopic Properties
3. Materials and Methods
3.1. Synthesis and Characterization
3.1.1. General
3.1.2. 1,3,5,7-Tetramethyl-2,6-dimethoxycarbonyl-8-(2-pyridyl)-BODIPY (2PyCO2Me)
3.1.3. 1,3,5,7-Tetramethyl-2,6-dimethoxycarbonyl-8-(3-pyridyl)-BODIPY (3PyCO2Me)
3.1.4. 1,3,5,7-Tetramethyl-2,6-dimethoxycarbonyl-8-(4-pyridyl)-BODIPY (4PyCO2Me)
3.1.5. 2-Nitro-1,3,5,7-tetramethyl-8-(2-pyridyl)-BODIPY (2PyNO2)
3.1.6. 2-Nitro-1,3,5,7-tetramethyl-8-(3-pyridyl)-BODIPY (3PyNO2)
3.1.7. 2-Nitro-1,3,5,7-tetramethyl-8-(4-pyridyl)-BODIPY (4PyNO2)
3.1.8. 2-Chloro-6-nitro-1,3,5,7-tetramethyl-8-(2-pyridyl)-BODIPY (2PyNO2Cl)
3.1.9. 2-Chloro-6-nitro-1,3,5,7-tetramethyl-8-(3-pyridyl)-BODIPY (3PyNO2Cl)
3.1.10. 2-Chloro-6-nitro-1,3,5,7-tetramethyl-8-(4-pyridyl)-BODIPY (4PyNO2Cl)
3.2. Spectroscopy Methods
3.3. X-ray Crystallography
3.4. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A


References
- Krumova, K.; Cosa, G. Bodipy Dyes with Tunable Redox Potentials and Functional Groups for Further Tethering: Preparation, Electrochemical, and Spectroscopic Characterization. J. Am. Chem. Soc. 2010, 132, 17560–17569. [Google Scholar] [CrossRef]
- Clarke, R.G.; Hall, M.J. Chapter Three—Recent Developments in the Synthesis of the BODIPY Dyes. Adv. Heterocycl. Chem. 2019, 128, 181–261. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, K. Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Gurubasavaraj, P.M.; Sajjan, V.P.; Muñoz-Flores, B.M.; Jiménez Pérez, V.M.; Hosmane, N.S. Recent Advances in BODIPY Compounds: Synthetic Methods, Optical and Nonlinear Optical Properties, and Their Medical Applications. Molecules 2022, 27, 1877. [Google Scholar] [CrossRef]
- Kaufman, N.E.; Meng, Q.; Griffin, K.E.; Singh, S.S.; Dahal, A.; Zhou, Z.; Fronczek, F.R.; Mathis, J.M.; Jois, S.D.; Vicente, M.G.H. Synthesis, Characterization, and Evaluation of near-IR Boron Dipyrromethene Bioconjugates for Labeling of Adenocarci-Nomas by Selectively Targeting the Epidermal Growth Factor Receptor. J. Med. Chem. 2019, 62, 3323–3335. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Yan, M.; Wang, X. Recent Progress of BODIPY Dyes With Aggregation-Induced Emission. Front. Chem. 2019, 7, 712. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent Advances of BODIPY Based Derivatives for Optoelectronic Applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Ray, C.; Schad, C.; Moreno, F.; Maroto, B.L.; Bañuelos, J.; Arbeloa, T.; García-Moreno, I.; Villafuerte, C.; Muller, G.; de la Moya, S. BCl3-Activated Synthesis of COO-BODIPY Laser Dyes: General Scope and High Yields under Mild Conditions. J. Org. Chem. 2020, 85, 4594–4601. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. Bodipy Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, M.; Ndung’U, C.; Bobadova-Parvanova, P.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis and Investigation of BODIPYs with Restricted Meso-8-Aryl Rotation. J. Porphyr. Phthalocyanines 2020, 24, 869–877. [Google Scholar] [CrossRef]
- Ortiz, M.J.; Garcia-Moreno, I.; Agarrabeitia, A.R.; Duran-Sampedro, G.; Costela, A.; Sastre, R.; Arbeloa, F.L.; Prieto, J.B.; Arbeloa, I.L. Red-Edge-Wavelength Finely-Tunable Laser Action from New BODIPY Dyes. Phys. Chem. Chem. Phys. 2010, 12, 7804–7811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bura, T.; Ziessel, R. Water-Soluble Phosphonate-Substituted BODIPY Derivatives with Tunable Emission Channels. Org. Lett. 2011, 13, 3072–3075. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y. A Water-Soluble Sulfonate-BODIPY Based Fluorescent Probe for Selective Detection of HOCl/OCl− in Aqueous Media. Analyst 2014, 139, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Urano, Y.; Fujikawa, Y.; Kobayashi, T.; Kojima, H.; Terai, T.; Hanaoka, K.; Nagano, T. Development of 2,6-Carboxy-Substituted Boron Dipyrromethene (BODIPY) as a Novel Scaffold of Ratiometric Fluorescent Probes for Live Cell Imaging. Chem. Commun. 2009, 45, 7015–7017. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Griffin, K.E.; Zhou, Z.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Syntheses of 1,2,3-Triazole-BODIPYs Bearing up to Three Carbohydrate Units. New J. Chem. 2018, 42, 8241–8246. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Vegesna, G.; Luo, F.-T.; Green, S.A.; Liu, H. Highly Water-Soluble Neutral BODIPY Dyes with Controllable Fluorescence Quantum Yields. Org. Lett. 2010, 13, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-W.; Li, M.; Shen, Z.; You, X.-Z. Meso-Pyridine Substituted Boron-Dipyrromethene (BDP) Dye as a PH Probe: Syn-Thesis, Crystal Structure and Spectroscopic Properties. Chin. J. Inorg. Chem. 2008, 24, 1247–1252. [Google Scholar]
- Zhou, Z.; Maki, T. Ratiometric Fluorescence Acid Probes Based on a Tetrad Structure Including a Single BODIPY Chromo-Phore. J. Org. Chem. 2021, 86, 17560–17566. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, T.; Fan, J.; Li, Z.; Jiang, N.; Wang, J.; Dou, B.; Sun, S.; Song, F.; Peng, X. A BODIPY-Based Fluorescent Dye for Mitochondria in Living Cells, with Low Cytotoxicity and High Photostability. Org. Biomol. Chem. 2013, 11, 555–558. [Google Scholar] [CrossRef]
- Raza, M.K.; Gautam, S.; Howlader, P.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A.R. Pyriplatin-Boron-Dipyrrome-Thene Conjugates for Imaging and Mitochondria-Targeted Photodynamic Therapy. Inorg. Chem. 2018, 57, 14374–14385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, B.L.; Situ, X.; Scholle, F.; Bartelmess, J.; Weare, W.W.; Ghiladi, R.A.A. Antifungal and Antibacterial Activities of a BODIPY-Based Photosensitizer. Molecules 2015, 20, 10604–10621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durantini, A.M.; Heredia, D.A.; Durantini, J.E.; Durantini, E.N. BODIPYs to the Rescue: Potential Applications in Photo-Dynamic Inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. [Google Scholar] [CrossRef]
- Xie, H.-R.; Gu, Y.-Q.; Liu, L.; Dai, J.-C. A H-Aggregating Fluorescent Probe for Recognizing Both Mercury and Copper Ions Based on a Dicarboxyl-Pyridyl Bifunctionalized Difluoroboron Dipyrromethene. New J. Chem. 2020, 44, 19713–19722. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, Y.J.; Lee, D.; Kim, B.-S.; Churchill, D.G. Real Nerve Agent Study Assessing Pyridyl Reactivity: Selective Fluo-Rogenic and Colorimetric Detection of Soman and Simulant. Sens. Actuators B 2017, 238, 145–149. [Google Scholar] [CrossRef]
- Luo, G.-G.; Fang, K.; Wu, J.-H.; Dai, J.-C.; Zhao, Q.-H. Noble-Metal-Free BODIPY-Cobaloxime Photocatalysts for Visible-Light-Driven Hydrogen Production. Phys. Chem. Chem. Phys. 2014, 16, 23884–23894. [Google Scholar] [CrossRef]
- Shen, X.-F.; Watanabe, M.; Takagaki, A.; Song, J.T.; Ishihara, T. Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocata-Lyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production. Catalysts 2020, 10, 535. [Google Scholar] [CrossRef]
- LaMaster, D.J.; Kaufman, N.E.M.; Bruner, A.S.; Vicente, M.G.H. Structure based modulation of electron dynamics in meso-(4-pyridyl)-BODIPYs: A computational and synthetic approach. J. Phys. Chem. A 2018, 122, 6372–6380. [Google Scholar] [CrossRef]
- Ndung’U, C.; LaMaster, D.; Dhingra, S.; Michel, N.H.; Bobadova-Parvanova, P.; Fronczek, F.R.; Elgrishi, N.; Vicente, M.G.H. A Comparison of the Photophysical and Electrochemical Properties of Meso-(2-, 3-, and 4-Pyridyl)-BODIPYs and Their Derivatives. Sensors 2022, 22, 5121. [Google Scholar] [CrossRef]
- Mula, S.; Ray, A.K.; Banerjee, M.; Chaudhuri, T.; Dasgupta, K.; Chattopadhyay, S. Design and Development of a New Pyrromethene Dye with Improved Photostability and Lasing Efficiency: Theoretical Rationalization of Photophysical and Photochemical Properties. J. Org. Chem. 2008, 73, 2146–2154. [Google Scholar] [CrossRef]
- Smith, N.W.; Dzyuba, S.V. Efficient Nitration of Meso-Tetraphenylporphyrin with Nitronium Tetrafluoroborate. Arkivoc 2010, 2010, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Liang, C.; Shirazian, S.; Zhou, Y.; Miller, T.; Cui, J.; Fukuda, J.Y.; Chu, J.-Y.; Nematalla, A.; Wang, X.; et al. Discovery of 5-[5-Fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic Acid (2-Diethylaminoethyl)amide, a Novel Tyrosine Kinase Inhibitor Targeting Vascular Endothelial and Platelet-Derived Growth Factor Receptor Tyrosine Kinase. J. Med. Chem. 2003, 46, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Weare, W.W.; Latortue, N.; Duong, C.; Jones, D.S. Meso-Pyridyl BODIPYs with Tunable Chemical, Optical and Electrochemical Properties. New J. Chem. 2013, 37, 2663–2668. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01, Computational Chemistry Software; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]




| Solvent | BODIPY | λabs (nm) | λem (nm) | Stokes Shift (nm) | Φf a | ε (M−1 cm−1) |
|---|---|---|---|---|---|---|
| CH3CN | 2Py b | 502 | 514 | 12 | 0.04 | 87,500 |
| 3Py b | 502 | 514 | 12 | 0.43 | 92,900 | |
| 4Py c | 501 | 515 | 14 | 0.31 | 72,100 | |
| 2PyCO2Me | 501 | 515 | 14 | 0.09 | 64,160 | |
| 3PyCO2Me | 501 | 512 | 11 | 0.61 | 87,690 | |
| 4PyCO2Me | 500 | 510 | 10 | 0.43 | 84,500 | |
| 2PyNO2 | 491 | 509 | 18 | 0.08 | 55,810 | |
| 3PyNO2 | 491 | 507 | 16 | 0.26 | 54,740 | |
| 4PyNO2 | 490 | 508 | 18 | 0.25 | 43,450 | |
| 2PyNO2/Cl | 512 | 527 | 15 | 0.13 | 23,790 | |
| 3PyNO2/Cl | 512 | 526 | 14 | 0.36 | 23,850 | |
| 4PyNO2/Cl | 511 | 525 | 14 | 0.28 | 16,510 | |
| CH3OH | 2PyCO2Me | 502 | 516 | 14 | 0.21 | 88,200 |
| 3PyCO2Me | 501 | 514 | 13 | 0.61 | 70,760 | |
| 4PyCO2Me | 501 | 516 | 15 | 0.39 | 72,880 | |
| 2PyNO2 | 491 | 511 | 20 | 0.09 | 64,750 | |
| 3PyNO2 | 491 | 510 | 19 | 0.13 | 587,240 | |
| 4PyNO2 | 491 | 510 | 19 | 0.12 | 52,160 | |
| 2PyNO2/Cl | 511 | 530 | 19 | 0.20 | 37,730 | |
| 3PyNO2/Cl | 511 | 529 | 18 | 0.35 | 51,110 | |
| 4PyNO2/Cl | 511 | 529 | 18 | 0.32 | 41,050 |
| BODIPY | λabs (nm) | Oscillator Strength | HOMO (eV) | LUMO (eV) | HOMO-LUMO Gap (eV) | Dipole Moment (D) | λem (nm) | Stokes Shift (nm) |
|---|---|---|---|---|---|---|---|---|
| 2Py a | 416 | 0.542 | −6.77 | −1.62 | 5.14 | 6.05 | 430 | 14 |
| 3Py a | 416 | 0.542 | −6.92 | −1.78 | 5.14 | 3.94 | 429 | 13 |
| 4Py a | 415 | 0.543 | −6.96 | −1.82 | 5.15 | 2.13 | 428 | 12 |
| 2PyCO2Me | 413 | 0.797 | −7.21 | −2.02 | 5.19 | 3.00 | 436 | 23 |
| 3PyCO2Me | 413 | 0.793 | −7.36 | −2.18 | 5.18 | 1.89 | 430 | 17 |
| 4PyCO2Me | 412 | 0.794 | −7.40 | −2.21 | 5.19 | 1.05 | 434 | 22 |
| 2PyNO2 | 408 | 0.655 | −7.44 | −2.22 | 5.21 | 9.51 | 436 | 28 |
| 3PyNO2 | 407 | 0.656 | −7.58 | −2.37 | 5.21 | 8.02 | 429 | 22 |
| 4PyNO2 | 406 | 0.660 | −7.62 | −2.40 | 5.22 | 7.54 | 431 | 25 |
| 2PyNO2Cl | 417 | 0.673 | −7.55 | −2.40 | 5.14 | 8.14 | 443 | 26 |
| 3PyNO2Cl | 416 | 0.675 | −7.69 | −2.55 | 5.14 | 6.44 | 438 | 21 |
| 4PyNO2Cl | 416 | 0.676 | −7.73 | −2.58 | 5.15 | 5.66 | 440 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndung’U, C.; Bobadova-Parvanova, P.; LaMaster, D.J.; Goliber, D.; Fronczek, F.R.; Vicente, M.d.G.H. 8(meso)-Pyridyl-BODIPYs: Effects of 2,6-Substitution with Electron-Withdrawing Nitro, Chloro, and Methoxycarbonyl Groups. Molecules 2023, 28, 4581. https://doi.org/10.3390/molecules28124581
Ndung’U C, Bobadova-Parvanova P, LaMaster DJ, Goliber D, Fronczek FR, Vicente MdGH. 8(meso)-Pyridyl-BODIPYs: Effects of 2,6-Substitution with Electron-Withdrawing Nitro, Chloro, and Methoxycarbonyl Groups. Molecules. 2023; 28(12):4581. https://doi.org/10.3390/molecules28124581
Chicago/Turabian StyleNdung’U, Caroline, Petia Bobadova-Parvanova, Daniel J. LaMaster, Dylan Goliber, Frank R. Fronczek, and Maria da Graça H. Vicente. 2023. "8(meso)-Pyridyl-BODIPYs: Effects of 2,6-Substitution with Electron-Withdrawing Nitro, Chloro, and Methoxycarbonyl Groups" Molecules 28, no. 12: 4581. https://doi.org/10.3390/molecules28124581