Isolation, Characterization, and Anti-Idiopathic Pulmonary Fibrosis Activity of a Fucoidan from Costaria costata
Abstract
:1. Introduction
2. Results and Discussion
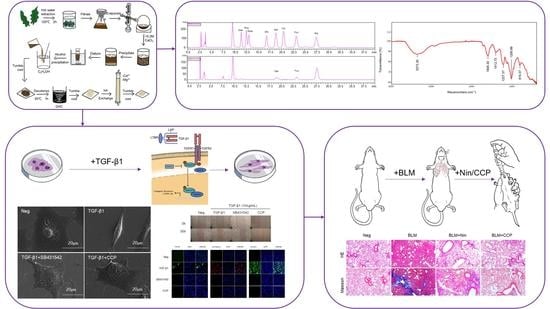
2.1. Chemical Properties of CCP
2.2. CCP Attenuated TGF-β1-Induced EMT in A549 Cells
2.3. CCP Attenuates EMT by Inhibiting Both TGF-β/Smad and PI3K/AKT/mTOR Signaling Pathway
2.4. CCP Attenuated Lung EMT Phenotype in a BLM-Induced Mice Model
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Costaria Costata Polysaccharides (CCP) Extraction and Analysis
3.2.1. CCP Extraction
3.2.2. Chemical Analysis
3.2.3. Determination of CCP-Molecular Weight
3.2.4. Monosaccharide Determination
3.2.5. Attenuated Total Refraction-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.3. Cell Culture and Treatment
3.4. Animal Models and Drug Treatment
3.5. Cellular Morphology Analysis
3.6. Cell Cytotoxicity Assay
3.7. Cell Wound-Healing Assay
3.8. HE and Masson Staining
3.9. Immunofluorescence Staining
3.10. Western Blot Analysis
3.11. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CCP | Costaria costata polysaccharide |
| TGF-β1 | Transforming growth factor-β1 |
| EMT | Epithelial-mesenchymal transition |
| COL2A1 | Collagen, type II, alpha 1 |
| BLM | Bleomycin |
| IPF | Idiopathic pulmonary fibrosis |
| ECM | Extracellular matrix |
| PMP | 1-phenyl-3-methyl-5-pyrazolone |
| DMSO | Dimethyl sulfoxide |
| MTT | 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide |
| ELISA | Enzyme-linked immunosorbent assay |
| IL-6 | Interleukin-6 |
| TPA | Total Phenol Assay |
References
- Srour, N.; Thébaud, B. Mesenchymal Stromal Cells in Animal Bleomycin Pulmonary Fibrosis Models: A Systematic Review. Stem Cells Transl. Med. 2015, 4, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.B.; Noble, P.W. Idiopathic pulmonary fibrosis. Orphanet J. Rare Dis. 2008, 3, 8. [Google Scholar] [CrossRef]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata Fucoidan on Expression of Matrix Metalloproteinase-1 Promoter, mRNA, and Protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; He, Y.; Ren, D.; Kow, F.; Qiao, Z.; Liu, S.; Yu, X. Structural characterisation of algae Costaria costata fucoidan and its effects on CCl4-induced liver injury. Carbohydr. Polym. 2014, 107, 247–254. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.H. Sublittoral ecology of kelp beds of the open coast area near Carmel, California. Biol. Bull. 1962, 122, 95–114. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- January, G.; Naidoo, R.; Kirby-McCullough, B.; Bauer, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517. [Google Scholar] [CrossRef]
- Li, S.S.; Wang, Q.K.; He, Y.H.; Ren, D.D.; Zhang, Z.Y. The extraction of serum lipids reducing function of fucoidan from seaweed Costaria costata. J. Dalian Ocean Univ. 2013, 28, 93–98. [Google Scholar]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Aguilera-Morales, M.; Casas-Valdez, M.; Carrillo-Domínguez, S.; González-Acosta, B.; Pérez-Gil, F. Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J. Food Compos. Anal. 2005, 18, 79–88. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.X.; Tang, Y.X.; Mao, J.L. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Monsur, H.A.; Jaswir, I.; Simsek, S.; Amid, A.; Alam, Z.; Tawakalit, A.-H. Cytotoxicity and inhibition of nitric oxide syntheses in LPS induced macrophage by water soluble fractions of brown seaweed. Food Hydrocoll. 2014, 42, 269–274. [Google Scholar] [CrossRef]
- Jin, W.; Wang, J.; Jiang, H.; Song, N.; Zhang, W.; Zhang, Q. The neuroprotective activities of heteropolysaccharides extracted from Saccharina japonica. Carbohydr. Polym. 2013, 97, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2012, 24, 715–723. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q.-L.; Chen, S.-T.; Tai, M.-R.; Cai, H.-Y.; Ding, R.; Liu, X.-F.; Chen, J.-P.; Luo, L.-X.; Zhong, S.-Y. Chemical Characterization and Immunomodulatory Activity of Fucoidan from Sargassum hemiphyllum. Mar. Drugs 2023, 21, 18. [Google Scholar] [CrossRef]
- Baek, A.R.; Lee, J.M.; Seo, H.J.; Park, J.S.; Lee, J.H.; Park, S.W.; Jang, A.S.; Kim, D.J.; Koh, E.S.; Uh, S.T.; et al. Apolipoprotein A1 Inhibits TGF-β1–Induced Epithelial-to-Mesenchymal Transition of Alveolar Epithelial Cells. Tuberc. Respir. Dis. 2016, 79, 143–152. [Google Scholar] [CrossRef]
- Han, M.-L.; Zhao, Y.-F.; Tan, C.-H.; Xiong, Y.-J.; Wang, W.-J.; Wu, F.; Fei, Y.; Wang, L.; Liang, Z.-Q. Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells. Acta Pharmacol. Sin. 2016, 37, 1606–1622. [Google Scholar] [CrossRef]
- Yuan, W.; Wei, F.; Ouyang, H.; Ren, X.; Hang, J.; Mo, X.; Liu, Z. CMTM3 suppresses chordoma progress through EGFR/STAT3 regulated EMT and TP53 signaling pathway. Cancer Cell Int. 2021, 21, 510. [Google Scholar] [CrossRef]
- Liu, S.; Ye, D.; Xu, D.; Liao, Y.; Zhang, L.; Liu, L.; Yu, W.; Wang, Y.; He, Y.; Hu, J.; et al. Autocrine epiregulin activates EGFR pathway for lung metastasis via EMT in salivary adenoid cystic carcinoma. Oncotarget 2016, 7, 25251–25263. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, Q.; Davis, F.; Mao, J.; Zhao, H.; Ma, D. Epithelial–mesenchymal transition in organ fibrosis development: Current understanding and treatment strategies. Burn. Trauma 2022, 10, tkac011. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Lu, D.-W.; Zhao, H.; Feng, Y.-L.; Chen, H.; Chen, D.-Q.; Vaziri, N.D.; Zhao, Y.-Y. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother. 2018, 101, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interactions 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, X.R.; Chung, A.C.; Hou, C.-C.; Lai, K.N.; Lan, H.Y. Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J. Pathol. 2010, 221, 390–401. [Google Scholar] [CrossRef]
- Naber, H.P.; Drabsch, Y.; Snaar-Jagalska, B.E.; ten Dijke, P.; van Laar, T. Snail and Slug, key regulators of TGF-β-induced EMT, are sufficient for the induction of single-cell invasion. Biochem. Biophys. Res. Commun. 2013, 435, 58–63. [Google Scholar] [CrossRef]
- Haslehurst, A.M.; Koti, M.; Dharsee, M.; Nuin, P.; Evans, K.; Geraci, J.; Childs, T.; Chen, J.; Li, J.; Weberpals, J.; et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 2012, 12, 91. [Google Scholar] [CrossRef]
- Larue, L.; Bellacosa, A. Epithelial–mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005, 24, 7443–7454. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wang, J.; Geng, L.; Yue, Y.; Deng, Z.; Wang, Q.; Zhang, Q. Low molecular weight fucoidan attenuating pulmonary fibrosis by relieving inflammatory reaction and progression of epithelial-mesenchymal transition. Carbohydr. Polym. 2021, 273, 118567. [Google Scholar] [CrossRef]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef]
- Liu, T.; Santos, F.G.D.L.; Phan, S.H. The Bleomycin Model of Pulmonary Fibrosis. Fibros. Methods Protoc. 2017, 1627, 27–42. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, F.; Sang, X.; Qiao, Y.; Shao, R.; Wang, Y.; Gao, S.; Fan, G.; Zhu, Y.; Yang, J. Combination therapy of tanshinone IIA and puerarin for pulmonary fibrosis via targeting IL6-JAK2-STAT3/STAT1 signaling pathways. Phytotherapy Res. 2021, 35, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gibbons, M.N. The determination of methylpentoses. Analyst 1955, 80, 268–276. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 578–582. [Google Scholar] [CrossRef]
- Davoren, M.; Herzog, E.; Casey, A.; Cottineau, B.; Chambers, G.; Byrne, H.J.; Lyng, F.M. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol. Vitr. 2007, 21, 438–448. [Google Scholar] [CrossRef]
- Yang, F.; Xiong, H.; Duan, L.; Li, Q.; Li, X.; Zhou, Y. MiR-1246 Promotes Metastasis and Invasion of A549 cells by Targeting GSK-3β–Mediated Wnt/β-Catenin Pathway. Cancer Res. Treat. 2019, 51, 1420–1429. [Google Scholar] [CrossRef]





| Yield (%) | Sulfate Content (%) | Fucose Content (%) | GlcA Content (%) | Protein Content (%) | Molecular Weight (Da) | Monosaccharide Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glc | Gal | Xyl | Fuc | ||||||
| 6.90 | 18.54 | 14.85 ± 0.13 | 13.20 ± 0.26 | 1.38 ± 0.06 | 149,846 | 0.244 | 0.111 | 0.073 | 0.042 | 0.906 | 0.03 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Geng, L.; Yu, H.; Wang, J.; Yue, Y.; Zhang, Q.; Wu, N. Isolation, Characterization, and Anti-Idiopathic Pulmonary Fibrosis Activity of a Fucoidan from Costaria costata. Molecules 2023, 28, 4343. https://doi.org/10.3390/molecules28114343
Wei S, Geng L, Yu H, Wang J, Yue Y, Zhang Q, Wu N. Isolation, Characterization, and Anti-Idiopathic Pulmonary Fibrosis Activity of a Fucoidan from Costaria costata. Molecules. 2023; 28(11):4343. https://doi.org/10.3390/molecules28114343
Chicago/Turabian StyleWei, Sijie, Lihua Geng, Haoyu Yu, Jing Wang, Yang Yue, Quanbin Zhang, and Ning Wu. 2023. "Isolation, Characterization, and Anti-Idiopathic Pulmonary Fibrosis Activity of a Fucoidan from Costaria costata" Molecules 28, no. 11: 4343. https://doi.org/10.3390/molecules28114343