Synthesis of a New Co Metal–Organic Framework Assembled from 5,10,15,20-Tetrakis((pyridin-4-yl) phenyl)porphyrin “Co-MTPhPyP” and Its Application to the Removal of Heavy Metal Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Ligand TPhPyP
2.2. Characterization of Co-MTPhPyP
2.2.1. FTIR Analysis
2.2.2. XPS Analysis
2.2.3. FESEM-EDS and DLS Analysis
2.2.4. XRD Analysis
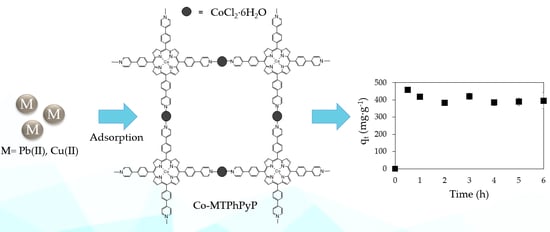
2.3. Adsorption of Co-MTPhPyP for Ions Pb(II) and Cu(II)
Kinetic Study for the Adsorption of Pb(II) with Co-MTPhPyP
3. Materials and Methods
3.1. Synthetic Procedures
3.2. Synthesis of 5,10,15,20-Tetrakis((Pyridin-4-yl) Phenyl)Porphyrin (TPhPyP)
3.3. Synthesis of Co-MTPhPyP
3.4. Adsorption Capacity of Co-MTPhPyP on Pb(II) and Cu(II)
3.5. Kinetic Analysis for the Sorption of Pb(II) by MTPhPyP-Co
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–Organic Frameworks (MOFs) for the Removal of Emerging Contaminants from Aquatic Environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Yu, W.; Luo, M.; Yang, Y.; Wu, H.; Huang, W.; Zeng, K.; Luo, F. Metal-Organic Framework (MOF) Showing Both Ultrahigh As(V) and As(III) Removal from Aqueous Solution. J. Solid State Chem. 2019, 269, 264–270. [Google Scholar] [CrossRef]
- Seyfi Hasankola, Z.; Rahimi, R.; Shayegan, H.; Moradi, E.; Safarifard, V. Removal of Hg2+ Heavy Metal Ion Using a Highly Stable Mesoporous Porphyrinic Zirconium Metal-Organic Framework. Inorg. Chim. Acta 2020, 501, 119264. [Google Scholar] [CrossRef]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–Organic Frameworks for Heavy Metal Removal from Water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Xu, G.R.; An, Z.H.; Xu, K.; Liu, Q.; Das, R.; Zhao, H.L. Metal Organic Framework (MOF)-Based Micro/Nanoscaled Materials for Heavy Metal Ions Removal: The Cutting-Edge Study on Designs, Synthesis, and Applications. Coord. Chem. Rev. 2021, 427, 213554. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Masoomi, M.Y.; Yamini, Y.; Morsali, A. Application of Mechanosynthesized Azine-Decorated Zinc(II) Metal-Organic Frameworks for Highly Efficient Removal and Extraction of Some Heavy-Metal Ions from Aqueous Samples: A Comparative Study. Inorg. Chem. 2015, 54, 425–433. [Google Scholar] [CrossRef]
- Bakhtiari, N.; Azizian, S. Adsorption of Copper Ion from Aqueous Solution by Nanoporous MOF-5: A Kinetic and Equilibrium Study. J. Mol. Liq. 2015, 206, 114–118. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of Hazardous Organics from Water Using Metal-Organic Frameworks (MOFs): Plausible Mechanisms for Selective Adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Rivera, J.M.; Rincón, S.; Ben Youssef, C.; Zepeda, A. Highly Efficient Adsorption of Aqueous Pb(II) with Mesoporous Metal-Organic Framework-5: An Equilibrium and Kinetic Study. J. Nanomater. 2016, 2016, 8095737. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, N.; Sharifnia, S.; Sheikh Arabi, M. A Porphyrin-Based Metal Organic Framework for High Rate Photoreduction of CO2 to CH4 in Gas Phase. J. CO2 Util. 2016, 16, 450–457. [Google Scholar] [CrossRef]
- Deibert, B.J.; Li, J. A Distinct Reversible Colorimetric and Fluorescent Low PH Response on a Water-Stable Zirconium–Porphyrin Metal–Organic Framework. Chem. Commun. 2014, 50, 9636–9639. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, A.; Eghbali, H.; Ardjmand, M.; Noufal, M.M.M.; Williamson, H.C.; Sayar, O. A Novel Bio-Compatible Sorbent Based on Carbon Nanostructure Modified by Porphyrin for Heavy Metal Separation from Industrial Wastewaters. J. Environ. Chem. Eng. 2016, 4, 398–404. [Google Scholar] [CrossRef]
- Bakhshayesh, S.; Dehghani, H. Synthesis of Magnetite-Porphyrin Nanocomposite and Its Application as a Novel Magnetic Adsorbent for Removing Heavy Cations. Mater. Res. Bull. 2013, 48, 2614–2624. [Google Scholar] [CrossRef]
- Fares, T.A.; Shady, M.E.; Awad, I.A. Fe/Co-MOF Nanocatalysts: Greener Chemistry Approach for the Removal of Toxic Metals and Catalytic Applications. ACS Omega 2022, 7, 23421–23444. [Google Scholar] [CrossRef]
- Feng, C.; Hua, F.-Z.; Lv, C.P.; Zhang, L.M.; Guo, J.-J.; Zhao, H. Highly stable supercapacitive performance of a (3, 4, 6-c)-connected 2D Co-MOF. Appl. Organomet. Chem. 2022, 36, e6684. [Google Scholar] [CrossRef]
- Ordaz, A.; Gil, E.; Hernández-Martínez, G.R.; Thalasso, F.; Rincón, S.; Zepeda, A. Microrespirometric Assessment of the Metal-Organic Framework [Co2(Btec)(Bipy)(DMF)2]: N (“MOF-Co”) to Prevent Inhibition by Arsenic in Activated Sludge. Environ. Sci. Water Res. Technol. 2020, 6, 1153–1162. [Google Scholar] [CrossRef]
- Lan, S.; Zhang, J.; Li, X.; Pan, L.; Li, J.; Wu, X.; Yang, S.T. Low Toxicity of Metal-Organic Framework MOF-74(Co) Nano-Particles In Vitro and In Vivo. Nanomaterials 2022, 12, 3398. [Google Scholar] [CrossRef]
- Bedel-cloutour, C.H.; Barois-gacherieu, C. Infrared Studies of Symmetrically and Asymmetrically Substituted Tetrapyrrolic Macrocycles: Free Bases and Indium Derivatives. Spectrochim. Acta 1988, 44A, 567–573. [Google Scholar] [CrossRef]
- Zhou, N.; Sun, Z.; Zhou, Q.; Lu, X.; Shao, H. Synthesis, Characterisation and Magnetic Behaviour of Ionic Metalloporphyrins: Metal–Tetrakis(N-Octyl-4-Pyridinium)–Porphyrins with Tetrabromoferrate(III) Anions. J. Chem. Res. 2013, 37, 445–450. [Google Scholar] [CrossRef]
- Tomita, K.; Shioya, N.; Shimoaka, T.; Wakioka, M.; Hasegawa, T. Control of supramolecular organizations by coordination bonding in tetrapyridylporphyrin thin films. Chem. Commun. 2022, 58, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, T.; Usuki, A.; Fukumori, K.; Ohta, T.; Ito, M.; Tatsumi, K. New Porphyrin-Based Metal-Organic Framework with High Porosity: 2-D Infinite 22.2-Å Square-Grid Coordination Network. Inorg. Chem. 2006, 45, 7988–7990. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, G.; Afzali, D.; Ghafainazari, A.; Saravani, H. Rapid Synthesis of Cobalt Metal Organic Framework. J. Inorg. Organomet. Polym. Mater. 2014, 24, 786–790. [Google Scholar] [CrossRef]
- Sengupta, A.; Datta, S.; Su, C.; Herng, T.S.; Ding, J.; Vittal, J.J.; Loh, K.P. Tunable Electrical Conductivity and Magnetic Property of the Two Dimensional Metal Organic Framework [Cu(TPyP)Cu2(O2CCH3)4]. ACS Appl. Mater. Interfaces 2016, 8, 16154–16159. [Google Scholar] [CrossRef]
- Wu, G.; Johnston, C.M.; MacK, N.H.; Artyushkova, K.; Ferrandon, M.; Nelson, M.; Lezama-Pacheco, J.S.; Conradson, S.D.; More, K.L.; Myers, D.J.; et al. Synthesis-Structure-Performance Correlation for Polyaniline-Me-C Non-Precious Metal Cathode Catalysts for Oxygen Reduction in Fuel Cells. J. Mater. Chem. 2011, 21, 11392–11405. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Wang, X.; Bao, Y.; Chen, W.; Niu, L. A Cobalt-Nitrogen Complex on N-Doped Three-Dimensional Graphene Framework as a Highly Efficient Electrocatalyst for Oxygen Reduction Reaction. Nanoscale 2014, 6, 15066–15072. [Google Scholar] [CrossRef]
- Jung, J.Y.; Hong, Y.L.; Kim, J.G.; Kim, M.J.; Kim, Y.K.; Kim, N.D. New Insight of Tailor-Made Graphene Oxide for the Formation of Atomic Co-N Sites toward Hydrogen Evolution Reaction. Appl. Surf. Sci. 2021, 563, 150254. [Google Scholar] [CrossRef]
- Xu, R.; Jian, M.; Ji, Q.; Hu, C.; Tang, C.; Liu, R.; Zhang, X.; Qu, J. 2D Water-Stable Zinc-Benzimidazole Framework Nanosheets for Ultrafast and Selective Removal of Heavy Metals. Chem. Eng. J. 2020, 382, 122658. [Google Scholar] [CrossRef]
- Lu, M.; Li, L.; Shen, S.; Chen, D.; Han, W. Highly Efficient Removal of Pb2+ by a Sandwich Structure of Metal-Organic Framework/GO Composite with Enhanced Stability. N. J. Chem. 2019, 43, 1032–1037. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-Supported Nanoscale Zero-Valent Iron: New Findings on Simultaneous Adsorption of Cd(II), Pb(II), and As(III) in Aqueous Solution and Soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Parvini, M.; Ghorbani, M. Adsorption of Heavy Metals (Cu2+ and Zn2+) on Novel Bifunctional Ordered Mesoporous Silica: Optimization by Response Surface Methodology. J. Taiwan Inst. Chem. Eng. 2018, 84, 123–141. [Google Scholar] [CrossRef]
- Abney, C.W.; Gilhula, J.C.; Lu, K.; Lin, W. Metal-Organic Framework Templated Inorganic Sorbents for Rapid and Efficient Extraction of Heavy Metals. Adv. Mater. 2014, 26, 7993–7997. [Google Scholar] [CrossRef]
- Shahbazi, A.; Younesi, H.; Badiei, A. Batch and Fixed-Bed Column Adsorption of Cu(II), Pb(II) and Cd(II) from Aqueous Solution onto Functionalised SBA-15 Mesoporous Silica. Can. J. Chem. Eng. 2013, 91, 739–750. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, S.; Chen, P.; Zhu, G.T.; Jiang, X.; Di, S. Adsorption Behavior and Mechanism of Pb(II) on a Novel and Effective Porphyrin-Based Magnetic Nanocomposite. Appl. Surf. Sci. 2019, 484, 124–134. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Liu, J.; Lei, X.; Zhao, X. Preparation of the Porphyrin-Functionalized Cotton Fiber for the Chromogenic Detection and Efficient Adsorption of Cd2+ Ions. J. Colloid Interface Sci. 2017, 488, 294–302. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy Metal Ion Removal of Wastewater by Zeolite-Imidazolate Frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J. A Simplified Synthesis for meso-tetraphenylporphin. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- León-Cedeño, F.; Menes-Arzate, M.; García-Ortega, H. Síntesis de 5,10,15,20-tetrafenilporfirina y sus derivados metálicos, experimento a microescala para el laboratorio de química orgánica heterocíclica. Rev. Cuba. De Química 2006, 18, 140–143. [Google Scholar]
- Lin, S.; Bediako, J.K.; Cho, C.W.; Song, M.H.; Zhao, Y.; Kim, J.A.; Choi, J.W.; Yun, Y.S. Selective Adsorption of Pd(II) over Interfering Metal Ions (Co(II), Ni(II), Pt(IV)) from Acidic Aqueous Phase by Metal-Organic Frameworks. Chem. Eng. J. 2018, 345, 337–344. [Google Scholar] [CrossRef]
- Abdollahi, N.; Akbar Razavi, S.A.; Morsali, A.; Hu, M.L. High Capacity Hg(II) and Pb(II) Removal Using MOF-Based Nanocomposite: Cooperative Effects of Pore Functionalization and Surface-Charge Modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions: Synthesis, applications and adsorption mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, T.; Xiong, M.; Sun, A.; Xu, Y.; Wu, Y.; Shu, W.; Xu, Z. Tuning adsorption capacity of metal–organic frameworks with Al3+ for phosphorus removal: Kinetics, isotherm and regeneration. Inorg. Chem. Commun. 2021, 132, 108804. [Google Scholar] [CrossRef]








| MOF | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|
| MOF-5 (Zn) | 658 [Pb2+] * | [10] |
| MIL-101 (Fe)/GO | 128.6 [Pb2+] * | [29] |
| Zn(Bim)(OAc) | 253.8 [Pb2+] * | [32] |
| 335.6 [Cu2+] * | ||
| MOF TMU-6 (Zn) | 224.0 [Pb2+] * 60.0 [Cu2+] * | [6] |
| MOF TMU-4 (Zn) | 237.0 [Pb2+] * 62.0 [Cu2+] * | |
| MOF TMU-5 (Zn) | 251.0 [Pb2+] * 57.0 [Cu2+] * | |
| Co-MTPhPyP | 383.4 [Pb2+] | This work |
| 168.0 [Cu2+] | ||
| Other Compounds | ||
| PAMAM-SBA-15 (polyamidoamine-SBA-15) | 242.4 [Pb2+] * | [33] |
| 110.5 [Cu2+] * | ||
| Zeolite-Nanoscale Zero Valent Iron (Z-NZVI) | 85.37 [Pb2+] * | [30] |
| Fe2O3@SBA-15-CS-APTMS | 107.3 [Cu2+] * | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arceo-Ruiz, H.; Xochitiotzi-Flores, E.; García-Ortega, H.; Farfán, N.; Santillan, R.; Rincón, S.; Zepeda, A. Synthesis of a New Co Metal–Organic Framework Assembled from 5,10,15,20-Tetrakis((pyridin-4-yl) phenyl)porphyrin “Co-MTPhPyP” and Its Application to the Removal of Heavy Metal Ions. Molecules 2023, 28, 1816. https://doi.org/10.3390/molecules28041816
Arceo-Ruiz H, Xochitiotzi-Flores E, García-Ortega H, Farfán N, Santillan R, Rincón S, Zepeda A. Synthesis of a New Co Metal–Organic Framework Assembled from 5,10,15,20-Tetrakis((pyridin-4-yl) phenyl)porphyrin “Co-MTPhPyP” and Its Application to the Removal of Heavy Metal Ions. Molecules. 2023; 28(4):1816. https://doi.org/10.3390/molecules28041816
Chicago/Turabian StyleArceo-Ruiz, Henry, Elba Xochitiotzi-Flores, Héctor García-Ortega, Norberto Farfán, Rosa Santillan, Susana Rincón, and Alejandro Zepeda. 2023. "Synthesis of a New Co Metal–Organic Framework Assembled from 5,10,15,20-Tetrakis((pyridin-4-yl) phenyl)porphyrin “Co-MTPhPyP” and Its Application to the Removal of Heavy Metal Ions" Molecules 28, no. 4: 1816. https://doi.org/10.3390/molecules28041816