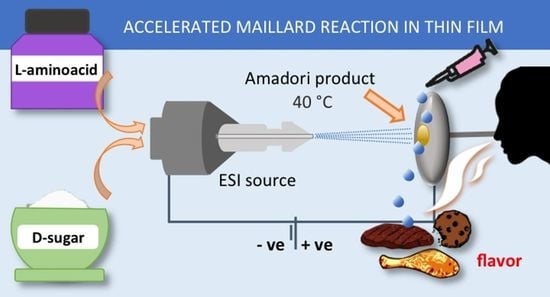
Kinetic Study of the Maillard Reaction in Thin Film Generated by Microdroplets Deposition
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effects of The ESI Z-Spray Desolvation Temperature
2.2. Maillard Reaction Kinetics in Thin Film with Respect to The Bulk Solution
3. Materials and Methods
3.1. Reagents
3.2. Mass Spectrometric Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coelho, F.; Eberlin, M.N. The Bridge Connecting Gas-Phase and Solution Chemistries. Angew. Chem. Int. Ed. 2011, 50, 5261–5263. [Google Scholar] [CrossRef]
- Eberlin, M.N. Electrospray Ionization Mass Spectrometry: A Major Tool to Investigate Reaction Mechanisms in Both Solution and the Gas Phase. Eur. J. Mass Spectrom. 2007, 13, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, M.N.; Cooks, R.G. Polar [4+ 2+] Diels-Alder cycloadditions of acylium ions in the gas phase. J. Am. Chem. Soc. 1993, 115, 9226–9233. [Google Scholar] [CrossRef]
- Gronert, S. Mass spectrometric studies of organic ion/molecule reactions. Chem. Rev. 2001, 101, 329–360. [Google Scholar] [CrossRef]
- Augusti, R.; Chen, H.; Eberlin, L.S.; Nefliu, M.; Cooks, R.G. Atmospheric pressure Eberlin transacetalization reactions in the heterogeneous liquid/gas phase. Int. J. Mass Spectrom. 2006, 253, 281–287. [Google Scholar] [CrossRef]
- Cooks, R.G.; Chen, H.; Eberlin, M.N.; Zheng, X.; Tao, W.A. Polar acetalization and transacetalization in the gas phase: The Eberlin reaction. Chem. Rev. 2006, 106, 188–211. [Google Scholar] [CrossRef]
- Salvitti, C.; Chiarotto, I.; Pepi, F.; Troiani, A. Charged-Tagged N-Heterocyclic Carbenes (NHCs): Revealing the Hidden Side of NHC-Catalysed Reactions through Electrospray Ionization Mass Spectrometry. ChemPlusChem 2021, 86, 209–223. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Cooks, R.G.; Yan, X. Accelerated Reaction Kinetics in Microdroplets: Overview and Recent Developments. Annu. Rev. Phys. Chem. 2020, 71, 31–51. [Google Scholar] [CrossRef]
- Tata, A.; Salvitti, C.; Pepi, F. From vacuum to atmospheric pressure: A review of ambient ion soft landing. Int. J. Mass Spectrom. 2020, 450, 116309. [Google Scholar] [CrossRef]
- Salvitti, C.; de Petris, G.; Troiani, A.; Managò, M.; Villani, C.; Ciogli, A.; Sorato, A.; Ricci, A.; Pepi, F. Accelerated D-Fructose Acid-Catalyzed Reactions in Thin Films Formed by Charged Microdroplets Deposition. J. Am. Soc. Mass Spectrom. 2022, 33, 565–572. [Google Scholar] [CrossRef]
- Chamberlayne, C.F.; Zare, R.N. Microdroplets can act as electrochemical cells. J. Chem. Phys. 2022, 156, 054705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.; Chen, H.; Zare, R.N. Accelerated Oxidation of Organic Sulfides by Microdroplet Chemistry. J. Org. Chem. 2021, 86, 5011–5015. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Jin, F.; Lee, J.K.; Zare, R.N. Aqueous microdroplets containing only ketones or aldehydes undergo Dakin and Baeyer-Villiger reactions. Chem. Sci. 2019, 10, 10974–10978. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mehari, T.F.; Wei, Z.; Liu, Y.; Cooks, R.G. Reaction acceleration at air-solution interfaces: Anisotropic rate constants for Katritzky transamination. J. Mass Spectrom. 2021, 56, 4585. [Google Scholar] [CrossRef]
- Basuri, P.L.; Gonzalez, E.; Morato, N.M.; Pradeep, T.; Cooks, R.G. Accelerated microdroplet synthesis of benzimidazoles by nucleophilic addition to protonated carboxylic acids. Chem. Sci. 2020, 11, 12686–12694. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, Z.; Nie, H.; Cooks, R.G. Reaction Acceleration Promoted by Partial Solvation at the Gas/Solution Interface. ChemPlusChem 2021, 86, 1362–1365. [Google Scholar] [CrossRef]
- Xiong, H.; Lee, J.K.; Zare, R.N.; Xiong, W.X. Strong electric field observed at the interface of aqueous microdroplets. J. Phys. Chem. Lett. 2020, 11, 7423–7428. [Google Scholar] [CrossRef]
- Narendra, N.; Chen, X.; Wang, J.; Charles, J.; Cooks, R.G.; Kubis, T. Quantum Mechanical Modeling of Reaction Rate Acceleration in Microdroplets. J. Phys. Chem. A 2020, 124, 4984–4989. [Google Scholar] [CrossRef]
- Chamberlayne, C.F.; Zare, R.N. Simple model for the electric field and spatial distribution of ions in a microdroplet. J. Chem. Phys. 2020, 152, 184702. [Google Scholar] [CrossRef]
- Pepi, F.; Ricci, A.; Garzoli, S.; Troiani, A.; Salvitti, C.; Di Rienzo, B.; Giacomello, P. A mass spectrometric study of the acid-catalysed D-fructose dehydration in the gas phase. Carbohydr. Res. 2015, 413, 145. [Google Scholar] [CrossRef]
- Ricci, A.; Di Rienzo, B.; Pepi, F.; Troiani, A.; Garzoli, S.; Giacomello, P. Acid-catalysed D-glucose dehydration in the gas phase: A mass spectrometric approach. J. Mass Spectrom. 2015, 50, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; de Petris, G.; Pepi, F.; Garzoli, S.; Salvitti, C.; Rosi, M.; Ricci, A. Base-assisted conversion of protonated D-fructose to 5-HMF: Searching for gas-phase green models. Chem. Open. 2019, 8, 1190. [Google Scholar] [CrossRef] [PubMed]
- Antonini, L.; Garzoli, S.; Ricci, A.; Troiani, A.; Salvitti, C.; Giacomello, P.; Ragno, R.; Patsilinakos, A.; Di Rienzo, B.; Pepi, F. Ab-initio and experimental study of pentose sugar dehydration mechanism in the gas phase. Carbohydr. Res. 2018, 19, 458–459. [Google Scholar] [CrossRef]
- Maillard, L.C. Action Des Acides Amine’s Sur Les Sucres. Formation Des Melanoidins Par Voie Methodique. Compt. Rend. 1912, 154, 66–68. [Google Scholar]
- Hodge, J.E. Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Chuyen, N.V. Maillard Reaction and food processing. Application aspects in Processed-Induced Chemical Changes in Food. Adv. Exp. Med. Biol. 1998, 434, 213–235. [Google Scholar] [CrossRef]
- Lane, M.J.; Nursten, H.E. Variety of odors produced in Maillard model systems and how they are influenced by reaction conditions, The Maillard Reaction in Foods and Nutrition. ACS Symp. Series. 1983, 215, 141–158. [Google Scholar]
- Fors, S. Sensory properties of volatile Maillard reaction products and related compounds. A literature review, The Maillard Reaction in Foods and Nutrition. ACS Symp. Series. 1983, 215, 185–286. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Dennis, J.W.; Granovsky, M.; Warren, C.E. Protein glycosylation in development and disease. BioEssays 1999, 21, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.B.; Marth, J.D. A Genetic Approach to Mammalian Glycan Function. Annu. Rev. Biochem. 2003, 72, 643–691. [Google Scholar] [CrossRef] [Green Version]
- Banazadeh, A.; Peng, W.; Veillon, L.; Mechref, Y. Carbon Nanoparticles and Graphene Nanosheets as MALDI Matrices in Glycomics: A New Approach to Improve Glycan Profiling in Biological Samples. J. Am.Soc. Mass Spectrom. 2018, 29, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 380. [Google Scholar] [CrossRef]
- Davidek, T.; Marabi, A.; Mauroux, O.; Bauwens, I.; Kraehenbuehl, K. Preparation of activated flavor precursor DFG, N-(1-deoxy-1-fructosylglycine) by combination of vacuum evaporation and closed system heating steps. Food Chem. 2018, 244, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Haase, P.T.; Kanzler, C.; Hildebrandt, J.; Kroh, L.W. Browning Potential of C 6-α-Dicarbonyl Compounds under Maillard Conditions. J. Agric. Food Chem. 2017, 65, 1924–1931. [Google Scholar] [CrossRef]
- Kanzler, C.; Schestkowa, H.; Haase, P.T.; Kroh, L.W. Formation of Reactive Intermediates, Color, and Antioxidant Activity in the Maillard Reaction of Maltose in Comparison to d-Glucose. J. Agric. Food Chem. 2017, 65, 8957–8965. [Google Scholar]
- Sun, A.; Wu, W.; Soladoye, O.P.; Aluko, R.E.; Bak, K.H.; Fu, Y.; Zhang, Y. Maillard reaction of food-derived peptides as a potential route to generate meat flavor compounds: A review. Food Res. Int. 2022, 151, 110823. [Google Scholar] [CrossRef]
- Sun, F.; Cui, H.; Zhan, H.; Xu, M.; Hayat, K.; Tahir, M.U.; Hussain, S.; Zhang, X.; Ho, C.T. Aqueous Preparation of Maillard Reaction Intermediate from Glutathione and Xylose and its Volatile Formation During Thermal Treatment. J. Food Sci. 2019, 84, 3584–3593. [Google Scholar] [CrossRef]
- Sasanam, S.; Rungsardthong, V.; Thumthanaruk, B.; Wijuntamook, S.; Rattananupap, V.; Vatanyoopaisarn, S.; Puttanlek, C.; Uttapap, D.; Mussatto, S.I. Properties and volatile profile of process flavorings prepared from D-xylose with glycine, alanine or valine by direct extrusion method. Food Bioscience 2021, 44, 101371–101382. [Google Scholar] [CrossRef]
- Ma, M.; Cui, H.; Wang, Z.; Hayat, K.; Jia, C.; Xu, Y.; Zhang, X.; Ho, C. Dependence and Conversion Mechanism for Selective Preparation of a Xylose-Diglycine Amadori Compound and a Cross-linking Product in an Aqueous Maillard Reaction. J. Agric. Food Chem. 2021, 69, 14915–14925. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, X.; Wang, J.; Zhang, S.; Zhang, X.; Cooks, R.G. High yield accelerated reactions in nonvolatile microthin films: Chemical derivatization for analysis of single-cell intracellular fluid. Chem. Sci. 2018, 9, 7779–7786. [Google Scholar] [CrossRef] [PubMed]
- Brands, C.M.J.; van Boekel, M.A.J.S. Reactions of Monosaccharides during Heating of Sugar-Casein Systems: Building of a Reaction Network Model. J. Agric. Food Chem. 2001, 49, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Brands, C.M.J.; van Boekel, M.A.J.S. Kinetic Modeling of Reactions in Heated Monosaccharide−Casein Systems. J. Agric. Food Chem. 2002, 50, 6725–6739. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Dalle Ore, F.; Benajiba, A.; Puigserver, A. Effects of pH on Caramelization and Maillard Reaction Kinetics in Fructose-Lysine Model Systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Xing, H.; Mossine, V.V.; Yaylayan, V. Diagnostic MS/MS fragmentation patterns for the discrimination between Schiff bases and their Amadori or Heyns rearrangement product. Carbohydr. Res. 2020, 491, 107985–107992. [Google Scholar] [CrossRef]
- Stamp, J.A.; Labuza, T.P. Kinetics of the Maillard reaction between aspartame and glucose in solution at high temperatures. J. Food Sci. 1983, 48, 543–544. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Guo, S.; Ma, S.; Yu, S.J. Kinetic modeling of Maillard reaction system subjected to pulsed electric field. Inn. Food Sci. Emerg. Techn. 2013, 20, 121–125. [Google Scholar] [CrossRef]
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, E.; Dufosse, L.; Guerard, F. Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- Wei, Z.; Wleklinski, M.; Ferreira, C.; Cooks, R.G. Reaction Acceleration in Thin Films with Continuous Product Deposition for Organic Synthesis. Angew. Chem. Int. Ed. 2017, 56, 9386–9390. [Google Scholar] [CrossRef]
- Salvitti, C.; Troiani, A.; Mazzei, F.; D’Agostino, C.; Zumpano, R.; Baldacchini, C.; Bizzarri, A.R.; Tata, A.; Pepi, F. The use of a commercial ESI Z-spray source for ambient ion soft landing and microdroplet reactivity experiments. Int. J. Mass Spectrom. 2021, 468, 116658–116665. [Google Scholar] [CrossRef]








| System | Slope (Thin Film) | Slope (Bulk) | RAF |
|---|---|---|---|
| D-xylose/L-glycine | 4.52 × 10−5 | 3.20 × 10−6 | 14.1 |
| D-xylose/L-lysine | 4.12 × 10−4 | 1.88 × 10−5 | 21.9 |
| D-glucose/L-glycine | 8.63 × 10−6 | - | ∞ |
| D-glucose/L-lysine | 1.23 × 10−4 | 2.71 × 10−6 | 45.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvitti, C.; de Petris, G.; Troiani, A.; Managò, M.; Ricci, A.; Pepi, F. Kinetic Study of the Maillard Reaction in Thin Film Generated by Microdroplets Deposition. Molecules 2022, 27, 5747. https://doi.org/10.3390/molecules27185747
Salvitti C, de Petris G, Troiani A, Managò M, Ricci A, Pepi F. Kinetic Study of the Maillard Reaction in Thin Film Generated by Microdroplets Deposition. Molecules. 2022; 27(18):5747. https://doi.org/10.3390/molecules27185747
Chicago/Turabian StyleSalvitti, Chiara, Giulia de Petris, Anna Troiani, Marta Managò, Andreina Ricci, and Federico Pepi. 2022. "Kinetic Study of the Maillard Reaction in Thin Film Generated by Microdroplets Deposition" Molecules 27, no. 18: 5747. https://doi.org/10.3390/molecules27185747