Potential Action Mechanism and Inhibition Efficacy of Morinda citrifolia Essential Oil and Octanoic Acid against Stagonosporopsis cucurbitacearum Infestations
Abstract
:1. Introduction
2. Results
2.1. Chromatographic Analysis of M. citrifolia EO
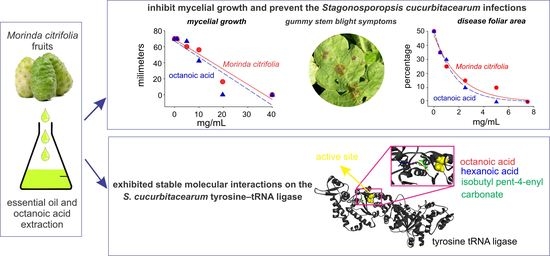
2.2. Interactions of Noni EO Components and Fungal Tyrosine–tRNA Ligases
2.3. Noni EO and Octanoic Acid Toxicity to S. cucurbitacearum Mycelial Growth and Melon Leaves
2.4. Evaluation of Preventive Controls of Noni EO in Melon Plants
3. Discussion
4. Materials and Methods
4.1. EO Extraction and Chemical Analysis
4.2. In Silico Studies of the Interactions among Noni EO Major Compounds and the Fungal Tyrosine–tRNA Ligase
4.2.1. Ligands and Modeling Targets
4.2.2. Molecular Docking Calculations
4.2.3. Molecular Dynamics Simulation
4.3. In Vitro Activities of Noni EO against S. cucurbitacearum
4.4. Toxicity and Physiological Parameters of the Melon Plants Treated with Noni EO
4.5. Preventive Control of S. cucurbitacearum by Noni EO
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duarte, J.A.D.; Fiaux, S.B.; Barbosa, E.; Toledo, P.F.S.; Silva, A.C.F.; Oliveira, E.E.; Leite, J.P.V.; Santos, M.G.; Rocha, L. Antifungal potential and biosafety of native plants from the Brazilian Restinga ecosystem. Clean. Eng. Technol. 2022, 8, 100493. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Osorio, P.R.A.; Leão, E.U.; Veloso, R.A.; Mourão, D.D.S.C.; Santos, G.R. Essential oils for alternative teak rust control. Floresta Ambiente 2018, 25, e20160391. [Google Scholar] [CrossRef]
- Osorio, P.R.A.; Dias, F.R.; Mourão, D.S.C.; Araujo, S.H.C.; Toledo, P.F.S.; Silva, A.C.F.; Viera, W.A.S.; Câmara, M.P.S.; Moura, W.S.; Aguiar, R.W.A.; et al. Essential oil of Noni, Morinda citrifolia L., fruits controls the rice stem-rot disease without detrimentally affect beneficial fungi and ladybeetles. Ind. Crops Prod. 2021, 170, 113728. [Google Scholar] [CrossRef]
- Holanda, L.; Bezerra, G.B.; Ramos, C.S. Potent Antifungal Activity of Essential Oil from Morinda Citrifolia Fruits Rich in Short-chain Fatty Acids. Int. J. Fruit Sci. 2020, 20 (Suppl. 2), S448–S454. [Google Scholar] [CrossRef]
- Dos Santos, P.R.; Alves, M.V.G.; dos Santos, G.R. Botanical and chemical fungicides in the treatment of commercial seeds of Brachiaria brizantha and Panicum maximum. J. Basic Microbiol. 2021, 61, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Kumar, A.; Suravajhala, R.; Bhagat, M. Bioactive potential of Cedrus deodara (Roxb.) Loud essential oil (bark) against Curvularia lunata and molecular docking studies. Appl. Sci. 2020, 2, 1045. [Google Scholar] [CrossRef]
- Luchi, N.; Ioos, R.; Santini, A. Fast and reliable molecular methods to detect fungal pathogens in woody plants. Appl. Microbiol. Biotechnol. 2020, 104, 2453–2468. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Van Laethem, S.; Frans, M.; Aerts, R.; Ceusters, J. pH modulation of the environment by Stagonosporopsis cucurbitacearum, an important pathogen causing fruit rot in Cucurbitaceae. Eur. J. Plant Pathol. 2021, 159, 235–245. [Google Scholar] [CrossRef]
- Stewart, J.E.; Turner, A.N.; Brewer, M.T. Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of cucurbits. Fungal Biol. 2015, 119, 370–382. [Google Scholar] [CrossRef]
- Keinath, A.P. From native plants in central Europe to cultivated crops worldwide: The emergence of Didymella bryoniae as a cucurbit pathogen. HortScience 2011, 46, 532–535. [Google Scholar] [CrossRef]
- Santos, G.; Leão, E.; de Castro, H.; do Nascimento, I.; Sarmento, R.D.A.; Sarmento-Brum, R. Gummy stem blight of watermelon: Etiology, epidemiology and control measures. J. Biotechnol. Biodivers. 2011, 2, 52–58. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Mol. Plant Pathol. 2021, 115, 101663. [Google Scholar] [CrossRef]
- Kefialew, Y.; Kunwar, S.; Abate, D.; Ayalew, A.; Colee, J.; Ritchie, L.; Olson, S.M.; Paret, M.L. Direct antifungal activity of tiadinil, a systemic acquired resistance inducer, and thymol formulations on Stagonosporopsis citrulli and control of watermelon gummy stem blight. J. Gen. Plant Pathol. 2018, 84, 284–295. [Google Scholar] [CrossRef]
- Santos, G.R.; Tschoeke, P.; Sarmento, R.; Oliveira, E.; Rodrigues-Silva, N.; Dalcin, M.; Haddi, K.; Silva, R. Impact of growing seasons and pesticides used on the occurrence and severity of the gummy stem blight in melon cultivation in Brazil. Eur. J. Plant Pathol. 2021, 161, 171–184. [Google Scholar] [CrossRef]
- Jones, J.G.; Korir, R.C.; Walter, T.L.; Everts, K.L. Reducing Chlorothalonil Use in Fungicide Spray Programs for Powdery Mildew, Anthracnose, and Gummy Stem Blight in Melons. Plant Dis. 2020, 104, 3213–3220. [Google Scholar] [CrossRef]
- Li, H.X.; Nuckols, T.A.; Harris, D.; Stevenson, K.L.; Brewer, M.T. Differences in fungicide resistance profiles and multiple resistance to a quinone-outside inhibitor (QoI), two succinate dehydrogenase inhibitors (SDHI), and a demethylation inhibitor (DMI) for two Stagonosporopsis species causing gummy stem blight of cucurbits. Pest Manag. Sci. 2019, 75, 3093–3101. [Google Scholar]
- Zhao, Q.; Wu, J.; Zhang, L.; Yan, C.; Jiang, S.; Li, Z.; Sun, D.; Lai, Y.; Gong, Z. Genome-scale analyses and characteristics of putative pathogenicity genes of Stagonosporopsis cucurbitacearum, a pumpkin gummy stem blight fungus. Sci. Rep. 2020, 10, 18065. [Google Scholar] [CrossRef]
- Li, H.-X.; Stevenson, K.L.; Brewer, M.T. Differences in sensitivity to a triazole fungicide among Stagonosporopsis species causing gummy stem blight of cucurbits. Plant Dis. 2016, 100, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Chaza, C.; Sopheak, N.; Mariam, H.; David, D.; Baghdad, O.; Moomen, B. Assessment of pesticide contamination in Akkar groundwater, northern Lebanon. Environ. Sci. Pollut. Res. 2018, 25, 14302–14312. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Costa, C.; Vesco, U.; Quaglia, G.; Guido, G. A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci. Total Environ. 2018, 615, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, F.; Eisvand, H.R. Effects of savory essential oil on germination parameters of Fusarium infected-seeds of wheat (Triticum aestivum L.). Iran. J. Plant Physiol. 2016, 6, 1745–1750. [Google Scholar]
- Wang, B.; Li, P.; Yang, J.; Yong, X.; Yin, M.; Chen, Y.; Feng, X.; Wang, Q. Inhibition efficacy of Tetradium glabrifolium fruit essential oil against Phytophthora capsici and potential mechanism. Ind. Crop. Prod. 2022, 176, 114310. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Ismail, S.; Man, C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012, 92, 593–597. [Google Scholar] [CrossRef]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.; Pereira, E.J.; Aguiar, R.W.; Oliveira, E.E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Radulović, N.; Mišić, M.; Aleksić, J.; Đoković, D.; Palić, R.; Stojanović, G. Antimicrobial synergism and antagonism of salicylaldehyde in Filipendula vulgaris essential oil. Fitoterapia 2007, 78, 565–570. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Ultee, A.; Slump, R.; Steging, G.; Smid, E. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.; Devi, R.; Ali, S.; Rao, D.; Sotheeswaran, S. Chemistry and Antimicrobial Activity of the Essential Oils from Ripe and Unripe fruits of the Fijian Morinda citrifolia (noni/kura) Rubiaceae. J. Essent. Oil-Bear. Plants 2008, 11, 598–602. [Google Scholar] [CrossRef]
- Andrade López, J.M.; Lanno, S.M.; Auerbach, J.M.; Moskowitz, E.C.; Sligar, L.A.; Wittkopp, P.J.; Coolon, J.D. Genetic basis of octanoic acid resistance in Drosophila sechellia: Functional analysis of a fine-mapped region. Mol. Ecol. 2017, 26, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Mayser, P. Medium chain fatty acid ethyl esters—Activation of antimicrobial effects by Malassezia enzymes. Mycoses 2015, 58, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short-and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Jelen, H.H.; Mildner, S.; Czaczyk, K. Influence of octanoic acid addition to medium on some volatile compounds and PR-toxin biosynthesis by Penicillium roqueforti. Lett. Appl. Microbiol. 2002, 35, 37–41. [Google Scholar] [CrossRef]
- Kishimoto, M.; Nakamura, K.; Tasaki, T.; Matsumoto, K.; Nakano, R.; Tanimoto, M. Fungal growth inhibition by cheese prepared using milk-clotting crude enzymes from the edible mushroom Hericium erinaceum. Food Sci. Technol. Res. 2020, 26, 93–99. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Fujita, T.; Kubo, I. Antifungal activity of alkanols against Zygosaccharomyces bailii and their effects on fungal plasma membrane. Phytother. Res. 2008, 22, 1349–1355. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Moumni, M.; Romanazzi, G.; Najar, B.; Pistelli, L.; Ben Amara, H.; Mezrioui, K.; Karous, O.; Chaieb, I.; Allagui, M.B. Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics 2021, 10, 104. [Google Scholar] [CrossRef]
- Oliveira, L.; Batista, A.; Fernandes, F.; Sales, G.; Nogueira, N. Antifungal activity and potential action mechanisms of essential oil of Ocimum gratissimum (Linn.) leaves against Candida species. Rev. Bras. Plantas Med. 2016, 18, 511–523. [Google Scholar] [CrossRef]
- Nogueira, J.H.; Gonçalez, E.; Galleti, S.R.; Facanali, R.; Marques, M.O.; Felício, J.D. Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. Int. J. Food Microbiol. 2010, 137, 55–60. [Google Scholar] [CrossRef]
- Jacob, K.S.; Ganguly, S.; Kumar, P.; Poddar, R.; Kumar, A. Homology model, molecular dynamics simulation and novel pyrazole analogs design of Candida albicans CYP450 lanosterol 14 α-demethylase, a target enzyme for antifungal therapy. J. Biomol. Struct. Dyn. 2017, 35, 1446–1463. [Google Scholar] [CrossRef]
- Chaudhary, M.; Kumar, N.; Baldi, A.; Chandra, R.; Arockia Babu, M.; Madan, J. Chloro and bromo-pyrazole curcumin Knoevenagel condensates augmented anticancer activity against human cervical cancer cells: Design, synthesis, in silico docking and in vitro cytotoxicity analysis. J. Biomol. Struct. Dyn. 2020, 38, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; Kong, Q.; Luo, S.; Wang, J.; Feng, S.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical composition, antioxidant, nntimicrobial, and phytotoxic potential of Eucalyptus grandis × E. urophylla leaves essential oils. Molecules 2021, 26, 1450. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Kainulainen, P.; Aflatuni, A.; Tiilikkala, K.; Holopainen, J.K. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: With special reference to limonene and its suitability for control of insect pests. Agric. Food Sci. Finl. 2001, 10, 243–259. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Chaimovitsh, D.; Shachter, A.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2017, 65, 19–30. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Karamaouna, F.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.; Polissiou, M.; Papatsakona, P.; Τsora, Ε.; Miller, T. Insecticidal activity of plant essential oils against the vine mealybug, Planococcus ficus. J. Insect Sci. 2013, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and Tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Campiglia, E.; Mancinelli, R.; Cavalieri, A.; Caporali, F. Use of essential oils of cinnamon, lavender and peppermint for weed control. Ital. J. Agron. 2007, 2, 171–178. [Google Scholar] [CrossRef]
- Schulz, M.; Kussmann, P.; Knop, M.; Kriegs, B.; Gresens, F.; Eichert, T.; Ulbrich, A.; Marx, F.; Fabricius, H.; Goldbach, H.; et al. Allelopathic monoterpenes interfere with Arabidopsis thaliana cuticular waxes and enhance transpiration. Plant Signal. Behav. 2007, 2, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kriegs, B.; Jansen, M.; Hahn, K.; Peisker, H.; Šamajová, O.; Beck, M.; Braun, S.; Ulbrich, A.; Baluška, F.; Schulz, M. Cyclic monoterpene mediated modulations of Arabidopsis thaliana phenotype: Effects on the cytoskeleton and on the expression of selected genes. Plant Signal. Behav. 2010, 5, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Damour, G.; Simonneau, T.; Cochard, H.; Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010, 33, 1419–1438. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- De, N.; Ram, D.; Pandey, S. Physiological traits as determinant of yield in muskmelon under field conditions. Indian J. Hortic 2008, 65, 40–43. [Google Scholar]
- Wang, F.; Qi, J.; Tian, M.; Gao, Y.; Xiong, X.; Wang, J.; Song, F.; Li, D. Genome sequence resource for Stagonosporopsis cucurbitacearum, a cause of gummy stem blight disease of watermelon. Mol. Plant-Microbe Interact. 2021, 34, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Ramachandran, G.T.; Sasisekharan, V. Conformation of polypeptides and proteins. Adv. Protein Chem. 1968, 23, 283–437. [Google Scholar] [PubMed]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous automated model evaluation (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins Struct. Funct. Genet. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Moura, W.; de Souza, S.R.; Campos, F.S.; Cangussu, A.S.R.; Santos, E.M.S.; Andrade, B.S.; Gomes, C.H.B.; Viana, K.F.; Haddi, K.; Oliveira, E.E. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crop. Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. The PyMOL Molecular Graphics System, Version 2.0; San Carlos, CA, USA, 2022.
- BIOVIA, Dassault Systèmes. Discovery Studio, Version 4.5; San Diego, CA, USA, 2022.
- Hospital, A.; Andrio, P.; Fenollosa, C.; Cicin-Sain, D.; Orozco, M.; Gelpí, J.L. MDWeb and MDMoby: An integrated web-based platform for molecular dynamics simulations. Bioinformatics 2012, 28, 1278–1279. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.S.; da Cruz, J.N.; Gomes Silva, S.; da Costa, W.A.; de Sousa, S.H.B.; Bezerra, F.W.F.; Teixeira, E.; da Silva, N.J.N.; de Aguiar Andrade, E.H.; de Jesus Chaves Neto, A.M.; et al. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J. Supercrit. Fluids 2019, 145, 74–84. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; da Cruz, J.N.; Almeida da Costa, W.; Silva, S.G.; Brito, M.d.P.; de Menezes, S.A.F.; de Jesus Chaves Neto, A.M.; de Aguiar Andrade, E.H.; de Carvalho Junior, R.N. Chemical composition, antimicrobial properties of Siparuna guianensis essential oil and a molecular docking and dynamics molecular study of its major chemical constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef]
- Dequech, S.; Ribeiro, L.d.P.; Sausen, C.; Egewarth, R.; Kruse, N. Fitotoxicidade causada por inseticidas botânicos em feijão-de-vagem (Phaseolus vulgaris L.) cultivado em estufa plástica. Rev. FZVA 2008, 15, 71–80. [Google Scholar]
- Santos, G.R.D.; Café-Filho, A.C.; Leão, F.F.; César, M.; Fernandes, L.E. Disease progress and crop losses due to watermelon gummy stem blight. Hort. Bras. 2005, 23, 228–232. [Google Scholar] [CrossRef]





| Peak | Compounds | RT a (min) | RI b | RI c | Peak Area (%) | Chemical Class | CAS Number |
|---|---|---|---|---|---|---|---|
| 1 | 2-hexanone,5-methyl- | 3.557 | 3.667 | 3.525 | 0.22 | Ketone | 110-12-3 |
| 2 | Hexanoic acid, methyl ester | 3.973 | 4.125 | 3.933 | 1.27 | Fatty acid | 106-70-7 |
| 3 | Hexanoic acid | 5.113 | 5.550 | 4.800 | 12.75 | Fatty acid | 142-62-1 |
| 4 | Benzene, tert-butyl- | 5.454 | 5.542 | 5.383 | 0.08 | Aromatic hydrocarbon | 98-06-6 |
| 5 | Butanoic acid,4-pentenyl ester | 6.012 | 6.125 | 5.983 | 0.19 | Fatty acid | 30563-31-6 |
| 6 | 2-Hexanone, 5-methyl- | 6.417 | 6.500 | 6.375 | 0.03 | Ketone | 110-12-3 |
| 7 | (E)-2-Methylbut-2-en-1-yl isobutyrate | 6.537 | 6.633 | 6.500 | 0.05 | Ester | 95654-17-4 |
| 8 | Octanoic acid, methyl ester | 6.363 | 7.058 | 6.825 | 2.91 | Fatty acid | 111-11-5 |
| 9 | Cyclopropane,1,2,3-trimethyl- | 7.333 | 7.408 | 7.242 | 0.07 | Hydrocarbon | 42984-19-0 |
| 10 | Octanoic acid | 8.130 | 8.642 | 7.625 | 75.77 | Fatty acid | 124-07-2 |
| 11 | Citronellol | 8.442 | 8.508 | 8.417 | 0.03 | Monoterpenoid | 106-22-9 |
| 12 | Hexanoic acid, 4-pentenyl ester | 8.861 | 9.008 | 8.767 | 2.57 | Fatty acid | 30563-33-8 |
| 13 | 1-Pentene, 5-(pentyloxy)- | 9.319 | 9.392 | 9.250 | 0.37 | Ether | 56052-88-1 |
| 14 | Pentane, 2,2′-oxybis- | 9.425 | 9.467 | 9.392 | 0.05 | Ether | 56762-00-6 |
| 15 | Decanoic acid, methyl ester | 9.719 | 9.783 | 9.683 | 0.15 | Fatty acid | 110-42-9 |
| 16 | Hexanoic acid, hexyl ester | 10.559 | 10.617 | 10.508 | 0.07 | Fatty acid | 6378-65-0 |
| 17 | Isobutyl pent-4-enyl carbonate | 11.490 | 11.825 | 11.433 | 3.12 | Carbonate | 0-00-0 |
| 18 | Dodecanoic acid, 2-penten-1-yl ester | 11.977 | 12.208 | 11.825 | 0.31 | Fatty acid | 0-00-0 |
| Total | 100 |
| Organism | Target | PDB Template | Identity (%) | Ramachandran Favored (%) | QMEAN |
|---|---|---|---|---|---|
| Stagonosporopsis cucurbitacearum | Tyrosine—tRNA ligase(EVM0001193.1) * | 5THH | 52.7% | 93.97% | 0.74 |
| Organism | Ligand | Affinity Energy (kcal/mol) |
|---|---|---|
| Stagonosporopsis cucurbitacearum | Octanoic acid | −5.1 |
| Isobutyl pent-4-enyl carbonate | −5.1 | |
| Hexanoic acid | −4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalcin, M.S.; Dias, B.L.; Viteri Jumbo, L.O.; Oliveira, A.C.S.S.; Araújo, S.H.C.; Moura, W.S.; Mourão, D.S.C.; Ferreira, T.P.S.; Campos, F.S.; Cangussu, A.S.R.; et al. Potential Action Mechanism and Inhibition Efficacy of Morinda citrifolia Essential Oil and Octanoic Acid against Stagonosporopsis cucurbitacearum Infestations. Molecules 2022, 27, 5173. https://doi.org/10.3390/molecules27165173
Dalcin MS, Dias BL, Viteri Jumbo LO, Oliveira ACSS, Araújo SHC, Moura WS, Mourão DSC, Ferreira TPS, Campos FS, Cangussu ASR, et al. Potential Action Mechanism and Inhibition Efficacy of Morinda citrifolia Essential Oil and Octanoic Acid against Stagonosporopsis cucurbitacearum Infestations. Molecules. 2022; 27(16):5173. https://doi.org/10.3390/molecules27165173
Chicago/Turabian StyleDalcin, Mateus S., Bruna L. Dias, Luis O. Viteri Jumbo, Ana C. S. S. Oliveira, Sabrina H. C. Araújo, Wellington S. Moura, Dalmarcia S. C. Mourão, Talita P. S. Ferreira, Fabricio S. Campos, Alex Sander R. Cangussu, and et al. 2022. "Potential Action Mechanism and Inhibition Efficacy of Morinda citrifolia Essential Oil and Octanoic Acid against Stagonosporopsis cucurbitacearum Infestations" Molecules 27, no. 16: 5173. https://doi.org/10.3390/molecules27165173