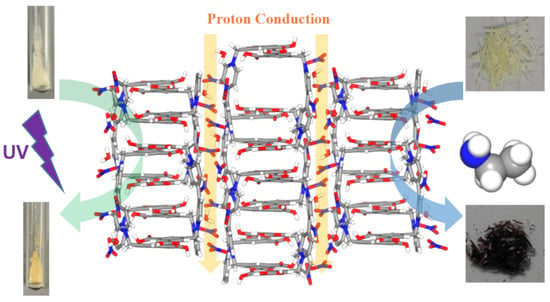
Multifunctional Viologen-Derived Supramolecular Network with Photo/Vapochromic and Proton Conduction Properties
Abstract
:1. Introduction
2. Results
2.1. Structural Description
2.2. Proton Conduction Properties
2.3. Photo/Vapochromic Properties
3. Materials and Methods
3.1. Materials and General Methods
3.2. Conductivity Measurements
3.3. Crystal Structure Determination
3.4. Synthesis of [H4bdcbpy(NO3)2·H2O] (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, M.-S.; Yang, C.; Wang, G.-E.; Xu, G.; Lv, X.-Y.; Xu, Z.-N.; Lin, R.-G.; Cai, L.-Z.; Guo, G.-C. A Room-Temperature X-ray-Induced Photochromic Material for X-ray Detection. Angew. Chem. Int. Ed. 2012, 51, 3432–3435. [Google Scholar] [CrossRef]
- Papadakis, R. Mono- and Di-Quaternized 4,4′-Bipyridine Derivatives as Key Building Blocks for Medium- and Environment-Responsive Compounds and Materials. Molecules 2020, 25, 1. [Google Scholar] [CrossRef] [Green Version]
- Kanj, A.B.; Chandresh, A.; Gerwien, A.; Grosjean, S.; Braese, S.; Wang, Y.; Dube, H.; Heinke, L. Proton-Conduction Photomodulation in Spiropyran-Functionalized MOFs with Large On-Off Ratio. Chem. Sci. 2020, 11, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Mercier, N. The Templating Effect and Photochemistry of Viologens in Halometalate Hybrid Crystals. Eur. J. Inorg. Chem. 2013, 1, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Tao, C.; Li, Y.; Li, J.; Yu, J. Methylviologen-Templated Zinc Gallophosphate Zeolitic Material with Dual Photo-/Thermochromism and Tuneable Photovoltaic Activity. Chem. Sci. 2015, 6, 2922–2927. [Google Scholar] [CrossRef] [Green Version]
- Hiroyoshi, K.; Tsuyoshi, S. Photochromism of Viologen Crystals. Bull. Chem. Soc. Jpn. 1987, 60, 794–796. [Google Scholar]
- Reus, C.; Stolar, M.; Vanderkley, J.; Nebauer, J.; Baumgartner, T. A Convenient N-Arylation Route for Electron-Deficient Pyridines: The Case of π-Extended Electrochromic Phosphaviologens. J. Am. Chem. Soc. 2015, 137, 11710–11717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Xing, F.; Bai, Y.-L.; Hu, M.; Zhao, Y.; Li, M.-X.; Zhu, S. High Sensitivity Viologen for a Facile and Versatile Sensor of Base and Solvent Polarity in Solution and Solid State in Air Atmosphere. Acs. Appl. Mater. Interfaces 2015, 7, 14493–14500. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kimura, M.; Shirai, H. Photo- and Thermo-chromism of A Ruthenium(II) Complex and Viologen-containing Polymer Film. Chem. Commun. 1997, 2061–2062. [Google Scholar] [CrossRef]
- Wu, J.; Tao, C.; Li, Y.; Yan, Y.; Li, J.; Yu, J. Methylviologen-Templated Layered Bimetal Phosphate: A Multifunctional X-ray-Induced Photochromic Material. Chem. Sci. 2014, 5, 4237–4241. [Google Scholar] [CrossRef]
- Sun, J.-K.; Yang, X.-D.; Yang, G.-Y.; Zhang, J. Bipyridinium Derivative-based Coordination Polymers: From Synthesis to Materials Applications. Coord. Chem. Rev. 2019, 378, 533–560. [Google Scholar] [CrossRef]
- Li, S.-L.; Han, M.; Zhang, Y.; Li, G.-P.; Li, M.; He, G.; Zhang, X.-M. X-ray and UV Dual Photochromism, Thermochromism, Electrochromism, and Amine-Selective Chemochromism in an Anderson-like Zn7 Cluster-Based 7-Fold Interpenetrated Framework. J. Am. Chem. Soc. 2019, 141, 12663–12672. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, L.; Yan, Y.; Shi, H.; Wang, B.; Liang, Z.; Li, J. A Novel Photo- and Hydrochromic Europium Metal–Organic Framework with Good Anion Sensing Properties. J. Mater. Chem. C. 2017, 5, 8999–9004. [Google Scholar] [CrossRef]
- Chen, C.; Sun, J.-K.; Zhang, Y.-J.; Yang, X.-D.; Zhang, J. Flexible Viologen-Based Porous Framework Showing X-ray Induced Photochromism with Single-Crystal-to-Single-Crystal Transformation. Angew. Chem. Int. Ed. 2017, 56, 14458–14462. [Google Scholar] [CrossRef]
- Sui, Q.; Li, P.; Yang, N.-N.; Gong, T.; Bu, R.; Gao, E.-Q. Differentiable Detection of Volatile Amines with a Viologen-Derived Metal–Organic Material. Acs. Appl. Mater. Interfaces 2018, 10, 11056–11062. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.-X.; Xuan, W.-M.; Zhang, H.; Tu, C.-Y.; Zhang, J. The Formation of a Hydrated Homochiral Helix from an Achiral Zwitterionic Salt, Spontaneous Chiral Symmetry Breaking and Redox Chromism of Crystals. Chem. Commun. 2009, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, J.; Zhao, G. Photochromism of Supramolecular Assemblies Based on Benzenecarboxylate Donors and Viologen Acceptors. New. J. Chem. 2019, 43, 6607–6614. [Google Scholar] [CrossRef]
- Yang, X.-D.; Chen, C.; Zhang, Y.-J.; Cai, L.-X.; Zhang, J. The Effects of Molecular Configuration on The Photochromism and Decolorization of Two Bipyridinium-Based Coordination Polymers. Inorg. Chem. Commun. 2015, 60, 122–125. [Google Scholar] [CrossRef]
- Shi, Z.-Q.; Ji, N.-N.; Wang, M.-H.; Li, G. A Comparative Study of Proton Conduction Between a 2D Zinc(II) MOF and Its Corresponding Organic Ligand. Inorg. Chem. 2020, 59, 4781–4789. [Google Scholar] [CrossRef]
- Xie, X.-X.; Yang, Y.-C.; Dou, B.-H.; Li, Z.-F.; Li, G. Proton Conductive Carboxylate-Based Metal–Organic Frameworks. Coord. Chem. Rev. 2020, 403, 213100. [Google Scholar] [CrossRef]
- Yang, W.; Yang, F.; Hu, T.-L.; King, S.C.; Wang, H.; Wu, H.; Zhou, W.; Li, J.-R.; Arman, H.D.; Chen, B. Microporous Diaminotriazine-Decorated Porphyrin-Based Hydrogen-Bonded Organic Framework: Permanent Porosity and Proton Conduction. Cryst. Growth Des. 2016, 16, 5831–5835. [Google Scholar] [CrossRef]
- Yang, L.-Z.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Fang, R.; Xu, C.-L.; Liu, W.-S. 2D Lanthanide MOFs Driven by a Rigid 3,5-Bis(3-Carboxy-Phenyl)Pyridine Building Block: Solvothermal Syntheses, Structural Features, and Photoluminescence and Sensing Properties. CrystEngComm 2016, 18, 6425–6436. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, X.; Kirillov, A.M.; Zhang, Y.; Hu, M.; Liu, W.; Yang, L.; Fang, R.; Liu, W. Covalent Construction of Sustainable Hybrid UiO-66-NH2@Tb-CP Material for Selective Removal of Dyes and Detection of Metal Ions. ACS. Sustain. Chem. Eng. 2019, 7, 3203–3212. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, D.; Meng, X.-D.; Pan, J.; Han, S.-D.; Xue, Z.-Z. Construction of Iodoargentates with Diverse Architectures: Template Syntheses, Structures, and Photocatalytic Properties. Cryst. Growth Des. 2020, 20, 1130–1138. [Google Scholar] [CrossRef]
- Gong, T.; Li, P.; Sui, Q.; Chen, J.-Q.; Xu, J.-H.; Gao, E.-Q. A Stable Electron-Deficient Metal-Organic Framework for Colorimetric and Luminescence Sensing of Phenols and Anilines. J. Mater. Chem. A. 2018, 6, 9236–9244. [Google Scholar] [CrossRef]
- Aulakh, D.; Varghese, J.R.; Wriedt, M. A New Design Strategy to Access Zwitterionic Metal–Organic Frameworks from Anionic Viologen Derivates. Inorg. Chem. 2015, 54, 1756–1764. [Google Scholar] [CrossRef]
- Sun, J.-K.; Tan, B.; Cai, L.-X.; Chen, R.-P.; Zhang, J.; Zhang, J. Polycatenation-Driven Self-Assembly of Nanoporous Frameworks Based on a 1D Ribbon of Rings: Regular Structural Evolution, Interpenetration Transformation, and Photochemical Modification. Chem. Eur. J. 2014, 20, 2488–2495. [Google Scholar] [CrossRef]
- Chand, S.; Pal, S.C.; Pal, A.; Ye, Y.; Lin, Q.; Zhang, Z.; Xiang, S.; Das, M.C. Metalo Hydrogen-Bonded Organic Frameworks (MHOFs) as New Class of Crystalline Materials for Protonic Conduction. Chem. Eur. J. 2019, 25, 1691–1695. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Unni, S.M.; Sharma, A.; Kurungot, S.; Ghosh, S.K. Two-in-One: Inherent Anhydrous and Water-Assisted High Proton Conduction in a 3D Metal-Organic Framework. Angew. Chem. Int. Ed. 2014, 53, 2638–2642. [Google Scholar] [CrossRef]
- Miyasaka, H. Control of Charge Transfer in Donor/Acceptor Metal-Organic Frameworks. Acc. Chem. Res. 2013, 46, 248–257. [Google Scholar] [CrossRef]
- Karmakar, A.; Illathvalappil, R.; Anothumakkool, B.; Sen, A.; Samanta, P.; Desai, A.V.; Kurungot, S.; Ghosh, S.K. Hydrogen-Bonded Organic Frameworks (HOFs): A New Class of Porous Crystalline Proton-Conducting Materials. Angew. Chem. Int. Ed. 2016, 55, 10667–10671. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-B.; Li, Y.-L.; Zhang, Z.-H.; Li, Z.-F.; Xiao, B.; Li, G. A Path to Improve Proton Conductivity: From a 3D Hydrogen-Bonded Organic Framework to a 3D Copper-Organic Framework. New. J. Chem. 2019, 43, 10637–10644. [Google Scholar] [CrossRef]
- Saravanabharathi, D.; Obulichetty, M.; Kumaravel, M. Facile Crystallization of 2-Phenyl Benzimidazole-5-Sulfonic Acid: Characterization of Lattice Water Dependent Zwitterionic Supramolecular Forms, with Modulation in Proton Conductivities. J. Chem. Sci. 2019, 131, 72. [Google Scholar] [CrossRef] [Green Version]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Proton Conductivity Control by Ion Substitution in a Highly Proton-Conductive Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 13166–13169. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-P.; Zhao, J.-J.; Liu, P.-Y.; Liu, Z.-L.; Wang, Y.-Q. A Metal-Organic Framework Constructed by a Viologen-Derived Ligand: Photochromism and Discernible Detection of Volatile Amine Vapors. New J. Chem. 2019, 43, 9032–9038. [Google Scholar] [CrossRef]
- Li, L.-K.; Li, H.-Y.; Li, T.; Quan, L.-H.; Xu, J.; Li, F.-A.; Zang, S.-Q. Photochromic and Photomodulated Luminescence Properties of Two Metal-Viologen Complexes Constructed by a Tetracarboxylate-Anchored Bipyridinium-Based Ligand. CrystEngComm 2018, 20, 6412–6419. [Google Scholar] [CrossRef]
- Guo, P.-Y.; Sun, C.; Zhang, N.-N.; Cai, L.-Z.; Wang, M.-S.; Guo, G.-C. Improving Coloration Time and Moisture Stability of Photochromic Viologen-Carboxylate Zwitterions. New J. Chem. 2018, 42, 15466–15471. [Google Scholar] [CrossRef]
- Guo, P.-Y.; Sun, C.; Zhang, N.-N.; Cai, L.-Z.; Wang, M.-S.; Guo, G.-C. An Inorganic-Organic Hybrid Photochromic Material with Fast Response to Hard and Soft X-rays at Room Temperature. Chem. Commun. 2018, 54, 4525–4528. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Lu, G.; Li, P.-X.; Lin, R.-G.; Cai, L.-Z.; Wang, M.-S.; Guo, G.-C. Influence of Supramolecular Interactions on Electron-Transfer Photochromism of the Crystalline Adducts of 4,4′-Bipyridine and Carboxylic Acids. Cryst. Growth Des. 2014, 14, 2527–2531. [Google Scholar] [CrossRef]
- Jin, X.-H.; Sun, J.-K.; Xu, X.-M.; Li, Z.-H.; Zhang, J. Conformational and Photosensitive Adjustment of The 4,4′-bipyridinium in Mn(II) Coordination Complexes. Chem. Commun. 2010, 46, 4695–4697. [Google Scholar] [CrossRef]
- Mehlana, G.; Bourne, S.A. Unravelling Chromism in Metal-Organic Frameworks. CrystEngComm 2017, 19, 4238–4259. [Google Scholar] [CrossRef]
- Wenger, O.S. Vapochromism in Organometallic and Coordination Complexes: Chemical Sensors for Volatile Organic Compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef]
- Li, X.-N.; Li, L.; Wang, H.-Y.; Fu, C.; Fu, J.-W.; Sun, Y.-N.; Zhang, H. A Novel Photochromic Metal-Organic Framework with Good Anion and Amine Sensing. Dalton Trans. 2019, 48, 6558–6563. [Google Scholar] [CrossRef]
- Tan, B.; Chen, C.; Cai, L.-X.; Zhang, Y.-J.; Huang, X.-Y.; Zhang, J. Introduction of Lewis Acidic and Redox-Active Sites into a Porous Framework for Ammonia Capture with Visual Color Response. Inorg. Chem. 2015, 54, 3456–3461. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, L.; Yan, Y.; Liu, Y.; Liang, Z.; Liu, Y.; Li, J. Metal-Organic Frameworks Based on Bipyridinium Carboxylate: Photochromism and Selective Vapochromism. J. Mater. Chem. C 2017, 5, 2084–2089. [Google Scholar] [CrossRef]
- Li, Z.-H.; Xue, L.-P.; Qin, Q.-P. A Methyl Miologen-Containing Cadmium Crystalline Material with Photochromism and Methylamine Sensing Properties. J. Solid State Chem. 2021, 293, 121755. [Google Scholar] [CrossRef]
- Yang, X.-D.; Zhu, R.; Yin, J.-P.; Sun, L.; Guo, R.-Y.; Zhang, J. Bipyridinium-Bearing Multi-stimuli Responsive Chromic Material with High Stability. Cryst. Growth Des. 2018, 18, 3236–3243. [Google Scholar] [CrossRef]
- Guo, M.-Y.; Li, P.; Yang, S.-L.; Bu, R.; Piao, X.-Q.; Gao, E.-Q. Distinct and Selective Amine- and Anion-Responsive Behaviors of an Electron-Deficient and Anion-Exchangeable Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 43958–43966. [Google Scholar] [CrossRef]







| Empirical Formula | C28 H24 N4 O15 |
|---|---|
| Formula weight | 656.51 |
| Temperature | 293(2) K |
| Wavelength | 0.71073 Å |
| Crystal system, space group | Monoclinic, P21/c |
| a = 16.653(3) Å α = 90° | |
| Unit cell dimensions | b = 13.379(2) Å β = 98.486(3)° |
| c = 24.101(4) Å γ = 90° | |
| Volume | 5310.9(15) Å3 |
| Z | 8 |
| Absorption coefficient | 1.36 mm−1 |
| F (000) | 2720 |
| Theta range for data collection | 1.26 to 28.31° |
| Limiting indices | −22 ≤ h ≤ 11, −17 ≤ k ≤ 17, −31 ≤ l ≤ 32 |
| Reflections collected/unique | 37,987 |
| Rint | 0.0943 |
| Completeness to theta = 28.31 | 99.4% |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9759 and 0.9720 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 13,137/4/851 |
| Goodness-of-fit on F2 | 1.004 |
| Final R indices [I > 2σ(I)] | R1 = 0.0859, wR2 = 0.2112 |
| R indices (all data) | R1 = 0.1813, wR2 = 0.2675 |
| Largest diff. peak and hole | 0.749 and −0.555 e Å−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Shi, H.; Zhang, C.; Yan, Y.; Liang, Z.; Li, J. Multifunctional Viologen-Derived Supramolecular Network with Photo/Vapochromic and Proton Conduction Properties. Molecules 2021, 26, 6209. https://doi.org/10.3390/molecules26206209
Zhang C, Shi H, Zhang C, Yan Y, Liang Z, Li J. Multifunctional Viologen-Derived Supramolecular Network with Photo/Vapochromic and Proton Conduction Properties. Molecules. 2021; 26(20):6209. https://doi.org/10.3390/molecules26206209
Chicago/Turabian StyleZhang, Chuanqi, Huaizhong Shi, Chenghui Zhang, Yan Yan, Zhiqiang Liang, and Jiyang Li. 2021. "Multifunctional Viologen-Derived Supramolecular Network with Photo/Vapochromic and Proton Conduction Properties" Molecules 26, no. 20: 6209. https://doi.org/10.3390/molecules26206209