Mechanism of Action of Endophytic Fungi Hypocrea lixii and Beauveria bassiana in Phaseolus vulgaris as Biopesticides against Pea Leafminer and Fall Armyworm
Abstract
:1. Introduction
2. Results
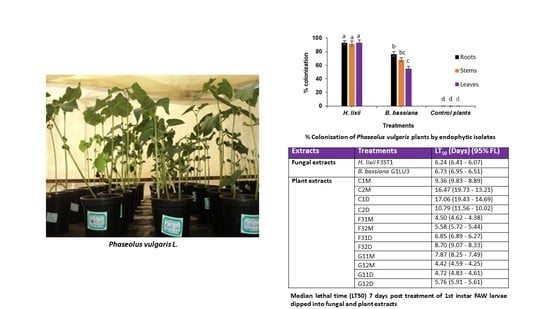
2.1. Colonization Assessment of Phaseolus vulgaris Plants Inoculated with Hypocrea lixii F3ST1 and Beauveria bassiana G1LU3
2.2. Organic Metabolites Characterized from Phaseolus vulgaris Plants
2.3. Effects of Plant Extracts on Pupation of 2nd Instar Liriomyza huidobrensis Larvae
2.4. Effects of Plant Extracts on Emergence of Liriomyza huidobrensis Adult Flies
2.5. Effects of Fungal and Plant Extracts on 1st Instar Fall armyworm, Spodoptera frugiperda Larvae
3. Discussion
4. Materials and Methods
4.1. Fungal Cultures, Suspensions Preparation and Viability Test
4.2. Seeds Inoculation and Endophytes Colonization Assessment
4.3. Insect Rearing and Treatments
4.4. Collection and Analysis of Volatiles
4.5. Solvent Liquid Extractions
4.6. Plant Extracts Bioassays
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Edwin, A.; Frenken, A. Regional Platform on Plant Pest and Diseases. Available online: https://docplayer.net/15483683-Regional-platform-on-plant-pest-and-diseases.html (accessed on 8 May 2021).
- Mitchell, D.C.; Lawrence, F.R.; Hartman, T.J.; Curran, J.M. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J. Am. Diet. Assoc. 2009, 109, 909–913. [Google Scholar] [CrossRef]
- Gathage, J.W.; Lagat, Z.O.; Fiaboe, K.K.M.; Akutse, K.S.; Ekesi, S.; Maniania, N.K. Prospects of fungal endophytes in the control of Liriomyza leafminer flies in common bean Phaseolus vulgaris under field conditions. BioControl 2016, 61, 741–753. [Google Scholar] [CrossRef]
- Abou-Fakhr Hammad, E.M.; Nemer, N.M. Population densities, spatial pattern and development of the pea leafminer (Diptera: Agromyzidae) on cucumber, swisschard and bean. J. Agric. Sci. 2000, 134, 61–68. [Google Scholar] [CrossRef]
- Chabi-Olaye, A.; Mwikya, N.M.; Fiaboe, K.K.M. Acceptability and suitability of three Liriomyza species as host for the endoparasitoid Phaedrotoma scabriventris: Implication for biological control of leafminers in the vegetable production system of Kenya. Biol. Control. 2013, 65, 1–5. [Google Scholar] [CrossRef]
- Green, J.M. The benefits of herbicide-resistant crops. Pest. Manag. Sci. 2012, 68, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Migiro, L.N.; Maniania, N.K.; Chabi-Olaye, A.; Vandenberg, J. Pathogenicity of entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) isolates to the adult pea leafminer (Diptera: Agromyzidae) and prospects of an autoinoculation device for infection in the field. Environ. Entomol. 2010, 39, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akutse, K.S.; Fiaboe, K.K.M.; Van Den Berg, J.; Ekesi, S.; Maniania, N.K. Effects of endophyte colonization of Vicia faba (fabaceae) plants on the life-history of leafminer parasitoids Phaedrotoma scabriventris (hymenoptera: Braconidae) and Diglyphus isaea (hymenoptera: Eulophidae). PLoS ONE 2014, 9, e109965. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Fiaboe, K.K.M.; Van den Berg, J.; Ekesi, S. Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol. 2013, 6, 293–301. [Google Scholar] [CrossRef]
- Muvea, A.M.; Meyhöfer, R.; Subramanian, S.; Poehling, H.M.; Ekesi, S.; Maniania, N.K. Colonization of onions by endophytic fungi and their impacts on the biology of thrips tabaci. PLoS ONE 2014, 9, e108242. [Google Scholar] [CrossRef] [Green Version]
- Mutune, B.; Ekesi, S.; Niassy, S.; Matiru, V.; Bii, C.; Maniania, N.K. Fungal endophytes as promising tools for the management of bean stem maggot Ophiomyia phaseoli on beans Phaseolus vulgaris. J. Pest. Sci. 2016, 89, 993–1001. [Google Scholar] [CrossRef]
- Akello, J.; Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control. 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Neven, L.G. The potential of the fungus, Muscodor albus, as a microbial control agent of potato tuber moth (Lepidoptera: Gelechiidae) in stored potatoes. J. Invertebr. Pathol. 2006, 91, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Martinuz, A.; Schouten, A.; Sikora, R.A. Systemically induced resistance and microbial competitive exclusion: Implications on biological control. Phytopathology 2012, 102, 260–266. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant. Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Menjivar, R.D.; Cabrera, J.A.; Kranz, J.; Sikora, R.A. Induction of metabolite organic compounds by mutualistic endophytic fungi to reduce the greenhouse whitefly Trialeurodes vaporariorum (Westwood) infection on tomato. Plant. Soil 2012, 352, 233–241. [Google Scholar] [CrossRef]
- Durango, D.; Pulgarin, N.; Echeverri, F.; Escobar, G.; Quiñones, W. Effect of salicylic acid and structurally related compounds in the accumulation of phytoalexins in cotyledons of common bean (Phaseolus vulgaris L.) cultivars. Molecules 2013, 18, 10609–10628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.H.; Zhang, Y.L.; Wang, L.W.; Wang, J.Y.; Zhang, C.L. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- De Backer, L.; Megido, R.C.; Fauconnier, M.L.; Brostaux, Y.; Francis, F.; Verheggen, F. Tuta absoluta-induced plant volatiles: Attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod. Plant. Interact. 2015, 9, 465–476. [Google Scholar] [CrossRef]
- Chokechaijaroenporn, O.; Bunyapraphatsara, N.; Kongchuensin, S. Mosquito repellent activities of ocimum volatile oils. Phytomedicine 1994, 1, 135–139. [Google Scholar] [CrossRef]
- Li, J.; Wakui, R.; Tebayashi, S.I.; Kim, C.S. Volatile attractants for the common bluebottle, Graphium sarpedon nipponum, from the host, Cinnamomum camphora. Biosci. Biotechnol. Biochem. 2010, 74, 1987–1990. [Google Scholar] [CrossRef] [Green Version]
- Hulshof, J.; Vanninen, I. Western flower thrips feeding on pollen, and its implications for control. In Thrips and Tospoviruses, Proceedings of the 7th International Symposium on Thysanoptera, Calabria, Italy, 2–7 July 2001; Australian National Insect Collection CSIRO: Canberra, Australia, 2002; Volume 7, pp. 173–179. [Google Scholar]
- Wei, J.N.; Zhu, J.; Kang, L. Volatiles released from bean plants in response to agromyzid flies. Planta 2006, 224, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [Green Version]
- Petrini, O.; Fisher, P.J. Fungal endophytes in Salicornia perennis. Trans. Br. Mycol. Soc. 1986, 87, 647–651. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M.; Schimmer, O. Modes of action of defensive secondary metabolites. Annu. Plant. Rev. 1999, 3, 17–133. [Google Scholar]
- Zhang, Q.; Yang, L.; Zhang, J.; Wu, M.; Chen, W.; Jiang, D.; Li, G. Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant. Soil 2015, 392, 101–114. [Google Scholar] [CrossRef]
- Aragüez, I.; Valpuesta Fernández, V. Metabolic engineering of aroma components in fruits. Biotechnol. J. 2013, 8, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Zeng, L.; Wang, T.T. Anti-fungal activity, mechanism studies on α-phellandrene and nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Azeem, M.; Rajarao, G.K.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Penicillium expansum volatiles reduce pine weevil attraction to host plants. J. Chem. Ecol. 2013, 39, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Arimura, G.I.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-β-ocimene and transcript accumulation of (E)-β-ocimene synthase in Lotus japonicus. Plant. Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef] [Green Version]
- Daisy, B.H.; Strobel, G.A.; Castillo, U.; Ezra, D.; Sears, J.; Weaver, D.K.; Runyon, J.B. Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 2002, 148, 3737–3741. [Google Scholar] [CrossRef] [Green Version]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant. Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant. Physiol. 1998, 116, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, S.; Saleem, M.; Siddique, S.; Ahmed, R.; Khanum, R.; Perveen, Z. Volatile components, antioxidant and antimicrobial activity of Citrus acida var. sour lime peel oil. J. Saudi Chem. Soc. 2009, 13, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.H.; Song, X.; Li, H.; Luo, T.; Dou, G.; Strobel, G. Antifungal activities of volatile secondary metabolites of four diaporthe strains isolated from Catharanthus roseus. J. Fungi 2018, 4, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manasa, C.; Nalini, M.S. L-Asparaginase Activity of Fungal Endophytes from Tabernaemontana heyneana Wall. (Apocynaceae), Endemic to the Western Ghats (India). Int. Sch. Res. Not. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Velásquez, A.; Valenzuela, M.; Carvajal, M.; Fiaschi, G.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant. Physiol. Biochem. 2020, 155, 437–443. [Google Scholar] [CrossRef]
- Mang, S.M.; Racioppi, R.; Camele, I.; Rana, G.L.; D’Auria, M. Use of volatile metabolite profiles to distinguish three Monilinia species. J. Plant. Pathol. 2015, 97, 55–59. [Google Scholar]
- Forti, L.; Cramarossa, M.R.; Filippucci, S.; Tasselli, G.; Turchetti, B.; Buzzini, P. Nonconventional yeast-promoted biotransformation for the production of flavor compounds. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 165–187. [Google Scholar]
- Menjivar, B.R.D. The Systemic Activity of Mutualistic Endophytic Fungi in Solanaceae and Cucurbitaceae Plants on the Behaviour of the Phloem-Feeding Insects Trialeurodes vaporariorum, Aphis Gossypii and Myzus Persicae. Available online: http://hss.ulb.uni-bonn.de/diss_online (accessed on 10 May 2021).
- Zhao, J.; Zhou, L.; Wang, J.; Shan, T.; Zhong, L.; Liu, X.; Gao, X. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 567–576. [Google Scholar]
- Goettel, M.S.; Inglis, D. Fungi: Hyphomycetes. Man. Tech. Insect Pathol. 1997, 213–249. [Google Scholar] [CrossRef]
- Schulz, B.; Guske, S.; Dammann, U.; Boyle, C. Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Dingle, J.; McGee, P.A. Some endophytic fungi reduce the density of pustules of Puccinia recondita f. sp. tritici in wheat. Mycol. Res. 2003, 107, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.; Denholm, I.; Ross, G.; Gorman, K. Relationship between bioassay data and the simulated field performance of insecticides against susceptible and resistant adult Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 1996, 86, 109–116. [Google Scholar] [CrossRef]
- Bates, D.; Bolker, B.; Christensen, R.H.B.; Singmann, H.; Grothendieck, G. Linear Mixed-Effects Models using “Eigen” and S4. R package. 2013. Available online: http://CRAN.R-project.org/package=lme4 (accessed on 10 September 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 265–267. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 8 May 2021).






| Compounds | Control Plants | LMF Damaged Control Plants | H. lixii Inoculated Plants | LMF Damaged H. lixii Inoculated Plants | B. bassiana Inoculated Plants | LMF Damaged B. bassiana Inoculated Plants |
|---|---|---|---|---|---|---|
| Retention time (Area percentage) | ||||||
| m-Cresol (1) | 13.29 (35.63) | - | 12.60 (72.92) | - | - | - |
| p-Cresol (2) | 13.63 (64.36) | - | 13.22 (13.28) | 13.20 (6.50) | 12.84 (0.74) | - |
| β-Phellandrene (3) | - | 11.86 (1.21) | - | - | - | - |
| α-Terpinene (4) | - | 12.35 (1.41) | - | - | - | 12.33 (0.94) |
| cis-Sabinene hydrate (5) | - | 12.51 (4.77) | - | - | - | 13.02 (3.73) |
| trans-Sabinene hydrate (6) | - | 13.04 (4.66) | - | - | - | - |
| Camphor (7) | - | 13.81 (1.56) | - | - | - | 13.81 (0.99 |
| Terpinen-4-ol (8) | - | 14.34 (28.68) | - | - | - | 14.32 (16.68) |
| (E)-Caryophyllene (9) | - | 17.84 (2.28) | - | 18.17 (1.23) | 17.81 (2.08) | - |
| Benzaldehyde, dimethyl acetal (10) | - | 13.25 (21.83) | - | 14.48 (0.47) | 13.20 (11.88) | 13.22 (16.51) |
| Heneicosane (11) | - | 15.87 (2.02) | - | 17.14 (1.49) | 21.02 (1.30) | 19.90 (4.53) |
| Butylated hydroxytoluene (12) | - | 18.93 (6.38) | - | 19.85 (3.32) | 18.89 (5.38) | 18.91 (4.64) |
| cis-1,1,3,5-Tetramethyl cyclohexane (13) | - | - | 9.46 (0.89) | - | - | - |
| Phenol (14) | - | - | 11.03 (1.47) | - | - | - |
| Benzyl alcohol (15) | - | - | 11.97 (6.88) | 8.23 (0.09) | - | - |
| 4-Methyloctane (16) | - | - | - | 8.23 (0.09) | - | - |
| 3-Methylanisole (17) | - | - | - | 11.93 (0.45) | - | - |
| (Z)-β-ocimene (18) | - | - | - | 11.92 (0.44) | 11.95 (0.31) | - |
| (E)-β-ocimene (19) | - | - | - | 12.13 (1.97) | 12.13 (1.41) | - |
| Naphthalene (20) | - | - | - | 14.50 (0.37) | 14.50 (0.37) | - |
| Methyl salicylate (21) | - | - | - | 14.68 (3.14) | 14.68 (4.47) | - |
| Heptadecane (22) | - | - | - | 15.89 (0.27) | - | - |
| 6-Propyl-tridecane (23) | - | - | - | 16.07 (0.44) | - | - |
| Propyl butanoate (24) | - | - | - | 16.68 (0.34) | - | - |
| Tridecane (25) | - | - | - | 17.39 (1.06) | - | - |
| α-Cedrene (26) | - | - | - | 17.73 (0.56) | 17.75 (0.57) | 17.77 (1.50) |
| Octadecane (27) | - | - | - | 17.82 (1.65) | - | - |
| Tetradecane (28) | - | - | - | 18.89 (4.86) | 17.42 (0.80) | - |
| Dibutyl phthalate (29) | - | - | - | 23.96 (1.17) | 24.02 (1.33) | - |
| 1-Methoxy-3-methylbenzene (17) | - | - | - | - | 11.66 (0.40) | - |
| (E)-γ-Bisabolene (30) | - | - | - | - | 18.81 (4.04) | - |
| 4,8,12-Trimethyl-1,3E,7E,11-tridecatetraene (31) | - | - | - | - | 19.68 (4.33) | - |
| Sulfurous acid, pentyl undecyl ester (32) | - | - | - | - | 19.86 (3.37) | - |
| Benzaldehyde (33) | - | - | - | - | - | 10.70 (0.61) |
| 5,7-Dimethyl undecane (34) | - | - | - | - | - | 15.85 (1.45) |
| 2-Methyl-2-ethyl-3-hydroxyhexyl propanoate (35) | - | - | - | - | - | 17.24 (0.77) |
| Extracts | Treatments | LT50 (Days) (95% FL) |
|---|---|---|
| Fungal extracts | H. lixii F3ST1 | 6.24 (6.41–6.07) |
| B. bassiana G1LU3 | 6.73 (6.95–6.51) | |
| Plant extracts | C1M | 9.36 (9.83–8.89) |
| C2M | 16.47 (19.73–13.21) | |
| C1D | 17.06 (19.43–14.69) | |
| C2D | 10.79 (11.56–10.02) | |
| F31M | 4.50 (4.62–4.38) | |
| F32M | 5.58 (5.72–5.44) | |
| F31D | 6.85 (6.89–6.27) | |
| F32D | 8.70 (9.07–8.33) | |
| G11M | 7.87 (8.25–7.49) | |
| G12M | 4.42 (4.59–4.25) | |
| G11D | 4.72 (4.83–4.61) | |
| G12D | 5.76 (5.91–5.61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebet, O.N.; Omosa, L.K.; Subramanian, S.; Nchiozem-Ngnitedem, V.-A.; Mmari, J.O.; Akutse, K.S. Mechanism of Action of Endophytic Fungi Hypocrea lixii and Beauveria bassiana in Phaseolus vulgaris as Biopesticides against Pea Leafminer and Fall Armyworm. Molecules 2021, 26, 5694. https://doi.org/10.3390/molecules26185694
Chebet ON, Omosa LK, Subramanian S, Nchiozem-Ngnitedem V-A, Mmari JO, Akutse KS. Mechanism of Action of Endophytic Fungi Hypocrea lixii and Beauveria bassiana in Phaseolus vulgaris as Biopesticides against Pea Leafminer and Fall Armyworm. Molecules. 2021; 26(18):5694. https://doi.org/10.3390/molecules26185694
Chicago/Turabian StyleChebet, Olivia Ngeno, Leonidah Kerubo Omosa, Sevgan Subramanian, Vaderament-A Nchiozem-Ngnitedem, John Onyari Mmari, and Komivi Senyo Akutse. 2021. "Mechanism of Action of Endophytic Fungi Hypocrea lixii and Beauveria bassiana in Phaseolus vulgaris as Biopesticides against Pea Leafminer and Fall Armyworm" Molecules 26, no. 18: 5694. https://doi.org/10.3390/molecules26185694