Influence of the Molecular Structure of Constituents and Liquid Phase Non-Ideality on the Viscosity of Deep Eutectic Solvents
Abstract
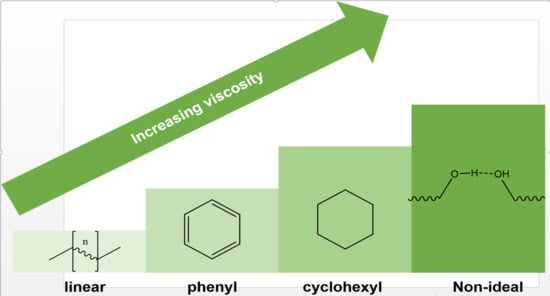
:1. Introduction
2. Results
2.1. Viscosity of Pure Substances
2.2. Viscosity of Eutectic Systems
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Horváth, I.T.; Anastas, P.T. Innovations and green chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef] [Green Version]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A search for natural hydrophobic deep eutectic solvents based on natural components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent advances in hydrophobic deep eutectic solvents for extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jung, D.; Park, K. Hydrophobic deep eutectic solvents for the extraction of organic and inorganic analytes from aqueous environments. TrAC Trends Anal. Chem. 2019, 118, 853–868. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Gębicki, J. Hydrophobic deep eutectic solvents in microextraction techniques–A review. Microchem. J. 2020, 152, 104384. [Google Scholar] [CrossRef]
- Almustafa, G.; Sulaiman, R.; Kumar, M.; Adeyemi, I.; Arafat, H.A.; AlNashef, I. Boron extraction from aqueous medium using novel hydrophobic deep eutectic solvents. Chem. Eng. J. 2020, 395, 125173. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of choline chloride-based deep eutectic solvents: Experiments and modeling. J. Chem. Eng. Data. 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Naser, J. Viscosity model for choline chloride-based deep eutectic solvents. Asia Pac. J. Chem. Eng. 2015, 10, 273–281. [Google Scholar] [CrossRef]
- Altamash, T.; Atilhan, M.; Aliyan, A.; Ullah, R.; Nasser, M.; Aparicio, S. rheological, thermodynamic, and gas solubility properties of phenylacetic acid-based deep eutectic solvents. Chem. Eng. Technol. 2017, 40, 778–790. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; Warrag, S.E.E.; Kroon, M.C. The curious case of hydrophobic deep eutectic solvents: A story on the discovery, design, and applications. ACS Sustain. Chem. Eng. 2020, 8, 10591–10612. [Google Scholar] [CrossRef]
- Lemaoui, T.; Darwish, A.S.; Attoui, A.; Abu Hatab, F.; Hammoudi, N.E.H.; Benguerba, Y.; Vega, L.F.; Alnashef, I.M. Predicting the density and viscosity of hydrophobic eutectic solvents: Towards the development of sustainable solvents. Green Chem. 2020, 22, 8511–8530. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Abranches, D.O.; Maximo, G.J.; Pinho, S.P.; Coutinho, J.A.P. Greener terpene–terpene eutectic mixtures as hydrophobic solvents. ACS Sustain. Chem. Eng. 2019, 7, 17414–17423. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, M.; McCourt, É.N.; Connolly, F.; Nockemann, P.; Swadźba-Kwaśny, M.; Holbrey, J.D. Hydrophobic deep eutectic solvents incorporating trioctylphosphine oxide: Advanced liquid extractants. ACS Sustain. Chem. Eng. 2018, 6, 17323–17332. [Google Scholar] [CrossRef] [Green Version]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From phase change materials to green solvents: Hydrophobic low viscous fatty acid–based deep eutectic solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Parvaneh, K.; Raeissi, S.; Shariati, A. A general viscosity model for deep eutectic solvents: The free volume theory coupled with association equations of state. Fluid Phase Equilib. 2018, 470, 193–202. [Google Scholar] [CrossRef]
- Al-Dawsari, J.N.; Bessadok-Jemai, A.; Wazeer, I.; Mokraoui, S.; AlMansour, M.A.; Hadj-Kali, M.K. Fitting of experimental viscosity to temperature data for deep eutectic solvents. J. Mol. Liq. 2020, 310, 113127. [Google Scholar] [CrossRef]
- Benguerba, Y.; Alnashef, I.M.; Erto, A.; Balsamo, M.; Ernst, B. A quantitative prediction of the viscosity of amine based DESs using Sσ-profile molecular descriptors. J. Mol. Struct. 2019, 1184, 357–363. [Google Scholar] [CrossRef]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Modeling of solid–liquid equilibria in deep eutectic solvents: A parameter study. Molecules 2019, 24, 2334. [Google Scholar] [CrossRef] [Green Version]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Design of deep eutectic systems: A simple approach for preselecting eutectic mixture constituents. Molecules 2020, 25, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Formation of glassy phases and polymorphism in deep eutectic solvents. J. Mol. Liq. 2020, 314, 113667. [Google Scholar] [CrossRef]
- Dow, R.B. The viscosity of mixtures of liquids at high pressures. Physics 1935, 6, 71–79. [Google Scholar] [CrossRef]
- Wang, X.; Sun, T.; Teja, A.S. density, viscosity, and thermal conductivity of eight carboxylic acids from (290.3 to 473.4) K. J. Chem. Eng. Data 2016, 61, 2651–2658. [Google Scholar] [CrossRef]
- Singh, R.P.; Sinha, C.P. Viscosities and activation energies of viscous flow of the binary mixtures of n-hexane with toluene, chlorobenzene and 1-hexanol. J. Chem. Eng. Data. 1984, 29, 132–135. [Google Scholar] [CrossRef]
- Assael, M.J.; Papadaki, M.; Wakeham, W.A. Measurements of the viscosity of benzene, toluene, and m-xylene at pressure up to 80 MPa. Int. J. Thermophys. 1991, 12, 449–457. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hosokawa, H.; Kubota, H.; Makita, T. Viscosity and density of binary mixtures of cyclohexane with n-octane, n-dodecane, and n-hexadecane under high pressures. Int. J. Thermophys. 1991, 12, 245–264. [Google Scholar] [CrossRef]
- Lowitz, D.A.; Spencer, J.W.; Webb, W.; Schiessler, R.W. Temperature-pressure-structure effects on the viscosity of several higher hydrocarbons. J. Chem. Phys. 1959, 30, 73–83. [Google Scholar] [CrossRef]
- Margerum, J.D.; Wong, S.M.; Jensen, J.E.; Van Ast, C.I.; Lackner, A.M. Effects of cyclohexyl groups and ortho-substituents on the viscosity of ester liquid crystal components. Mol. Cryst. Liq. Cryst. 1985, 122, 97–109. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Crespo, E.A.; Pontes, P.V.A.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.C.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Tunable hydrophobic eutectic solvents based on terpenes and monocarboxylic acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846. [Google Scholar] [CrossRef]
- Xin, K.; Roghair, I.; Gallucci, F.; van Sint Annaland, M. Total vapor pressure of hydrophobic deep eutectic solvents: Experiments and modelling. J. Mol. Liq. 2021, 325, 115227. [Google Scholar] [CrossRef]
- Angell, C.A. Liquid fragility and the glass transition in water and aqueous solutions. Chem. Rev. 2002, 102, 2627–2650. [Google Scholar] [CrossRef]
- Shao, X.; Yang, S.; Chen, C.; Fan, L.; Yu, Z. Temperature-dependent rheological behaviors of binary eutectic mixtures of sugar alcohols for latent heat storage: A comparative study with pure sugar alcohols. J. Therm. Sci. 2021. [Google Scholar] [CrossRef]
- Baird, J.A.; Santiago-Quinonez, D.; Rinaldi, C.; Taylor, L.S. Role of viscosity in influencing the glass-forming ability of organic molecules from the undercooled melt state. Pharm. Res. 2012, 29, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Harada, T.; Yoshihashi, Y.; Yonemochi, E.; Terada, K.; Moriyama, H. Correlation between glass-forming ability and fragility of pharmaceutical compounds. J. Phys. Chem. B 2015, 119, 4873–4880. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Müller, S.; González de Castilla, A.; Gurikov, P.; Matias, A.A.; Bronze, M.D.; Fernández, N. Physicochemical characterization and simulation of the solid–liquid equilibrium phase diagram of terpene-based eutectic solvent systems. Molecules 2021, 26, 1801. [Google Scholar] [CrossRef]
- Acree, W.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds. Sublimation, vaporization and fusion enthalpies from 1880 to 2015. Part 1. C1−C10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]










| Name | Structure | Supplier | Declared Purity/% | Water Content/mg·g−1 | Tm/K |
|---|---|---|---|---|---|
| l-Menthol |  | Sigma Aldrich | ≥99 | 0.1075 | 314.6 [23] |
| Thymol |  | Sigma Aldrich | >99 | 0.0869 | 322.7 [24] |
| Carvacrol |  | Sigma Aldrich | >99 | 5.9269 | 274.2 [24] |
| Caprylic acid |  | Merck | 99 | 1.2530 | 288.0 [23] |
| Capric acid |  | Alfa Aesar | 99 | 0.1670 | 303.9 [23] |
| Lauric acid |  | Merck | 99 | 0.0842 | 316.6 [23] |
| 2-Phenylacetic acid |  | Alfa Aesar | ≥99 | 0.0656 | 349.2 [39] |
| 3-Phenylpropionic acid |  | Alfa Aesar | 99 | 0.0504 | 321.6 [23] |
| 4-Phenylbutyric acid |  | Alfa Aesar | >99 | 0.0779 | 324.2 [39] |
| Cyclohexanecarboxylic acid |  | ThermoFisher | 98 | 2.2280 | 299.4 [23] |
| 2-Cyclohexylacetic acid |  | Alfa Aesar | ≥98 | 0.7425 | 302.6 [39] |
| 3-Cyclohexylpropionic acid |  | ThermoFisher | >98 | 1.2636 | 291.3 [23] |
| Constituents | xe, menthol | Te/K | ||
|---|---|---|---|---|
| Experimental | Calculated * | Experimental | Calculated * | |
| Ideal eutectic systems | ||||
| l-Menthol + caprylic acid | 0.50 [23] | 0.42 | 266.7 [23] | 270.1 |
| l-Menthol + capric acid | 0.60 [23] | 0.56 | 279.7 [23] | 283.2 |
| l-Menthol + lauric acid | 0.70 [23] | 0.67 | 289.5 [23] | 292.5 |
| l-Menthol + 2-phenylacetic acid | – | 0.65 | 290.8 | – |
| l-Menthol + 3-phenylpropionic acid | 0.60 [23] | 0.55 | 283.1 [23] | 282.1 |
| l-Menthol + 4-phenylbutyric acid | – | 0.61 | 287.2 | – |
| l-Menthol + cyclohexanecarboxylic acid | 0.50 [23] | 0.40 | 265.0 [23] | 267.7 |
| l-Menthol + 2-cyclohexylacetic acid | – | 0.45 | 272.9 | – |
| l-Menthol + 3-cyclohexylpropionic acid | 0.50 [23] | 0.42 | 262.1 [23] | 270.2 |
| Thymol + caprylic acid | 0.35 [32] | 0.29 | 275.0 [32] | 277.5 |
| Thymol + 3-phenylpropionic acid | – | 0.44 | 291.6 | – |
| Thymol + cyclohexanecarboxylic acid | – | 0.29 | 277.6 | – |
| Non-ideal eutectic systems | ||||
| l-Menthol + thymol | 0.57 [24] | 0.61 | 241.5 [24] | 287.5 |
| l-Menthol + carvacrol | 0.42 [24] | 0.30 | 243.3 [24] | 256.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhadid, A.; Mokrushina, L.; Minceva, M. Influence of the Molecular Structure of Constituents and Liquid Phase Non-Ideality on the Viscosity of Deep Eutectic Solvents. Molecules 2021, 26, 4208. https://doi.org/10.3390/molecules26144208
Alhadid A, Mokrushina L, Minceva M. Influence of the Molecular Structure of Constituents and Liquid Phase Non-Ideality on the Viscosity of Deep Eutectic Solvents. Molecules. 2021; 26(14):4208. https://doi.org/10.3390/molecules26144208
Chicago/Turabian StyleAlhadid, Ahmad, Liudmila Mokrushina, and Mirjana Minceva. 2021. "Influence of the Molecular Structure of Constituents and Liquid Phase Non-Ideality on the Viscosity of Deep Eutectic Solvents" Molecules 26, no. 14: 4208. https://doi.org/10.3390/molecules26144208