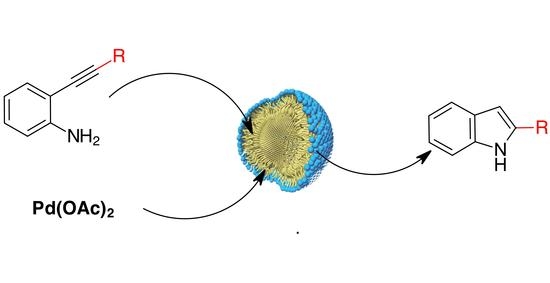
Synthesis of 2-Substitued Indoles via Pd-Catalysed Cyclization in an Aqueous Micellar Medium
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Herraiz-Cobo, J.; Albericio, F.; Álvarez, M. The Larock Reaction in the Synthesis of Heterocyclic Compounds. Adv. Heterocycl. Chem. 2015, 116, 1–35. [Google Scholar] [CrossRef]
- Sinha, A.K.; Equbal, D.; Uttam, M.R. Metal-catalyzed privileged 2- and 3-functionalized indole synthesis. Chem. Heterocycl. Compd. 2018, 54, 292–301. [Google Scholar] [CrossRef]
- Colella, M.; Degennaro, L.; Luisi, R. Continuous Flow Synthesis of Heterocycles: A Recent Update on the Flow Synthesis of Indoles. Molecules 2020, 25, 3242. [Google Scholar] [CrossRef]
- Plenio, H. Catalysts for the Sonogashira Coupling-The Crownless Again Shall Be King. Angew. Chem. Int. Ed. 2008, 47, 6954–6956. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Hiroya, K.; Itoh, S.; Sakamoto, T. Mild and efficient cyclization reaction of 2-ethynylaniline derivatives to indoles in aqueous medium. Tetrahedron 2005, 61, 10958–10964. [Google Scholar] [CrossRef]
- Hiroya, K.; Itoh, S.; Sakamoto, T. Development of an Efficient Procedure for Indole Ring Synthesis from 2-Ethynylaniline Derivatives Catalyzed by Cu(II) Salts and Its Application to Natural Product Synthesis. J. Org. Chem. 2004, 69, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Sasaki, I.; Hirai, Y.; Namba, K.; Imagawa, H.; Nishizawa, M. Silaphenylmercuric Triflate Catalyzed Reactions: Synthesis of a Solid-Supported Mercuric Salt Catalyst. Angew. Chem. Int. Ed. 2009, 48, 1244–1247. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, W.; Chai, Z.; Zhao, G. Et2Zn-Catalyzed Intramolecular Hydroamination of Alkynyl Sulfonamides and the Related Tandem Cyclization/Addition Reaction. J. Org. Chem. 2007, 72, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Marsiciano, V.; Arcadi, A.; Chiarini, M.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A. Synthesis of functionalised 2-3,dihydroquinolin-4(1H)-ones vs.quinoline or N-alkenylindole derivates through sequential reaction of 2-alkynylanilines with ketones. Org. Biomol. Chem. 2021, 19, 421–438. [Google Scholar] [CrossRef]
- Mancuso, R.; Dalpozzo, R. Recent Progress in the Transition Metal Catalyzed Synthesis of Indoles. Catalysts 2018, 8, 458. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.; Anand, R.V. Expedient Access to Unsymmetrical Diarlyindoliylmethanes through Palladium-Catalyzed Domino Elettrophilic Cyclization -Extended Conjugate Addition Approch. Org. Lett. 2015, 17, 3390–3393. [Google Scholar] [CrossRef]
- Arcadi, A.; Bianchi, G.; Inesi, A.; Marinelli, F.; Rossi, L. Electrochemical-mediated cyclization of 2-alkynylanilines: A clean and safe synthesis of indole derivatives. Eur. J. Org. Chem. 2008, 783–787. [Google Scholar] [CrossRef]
- Carpita, A.; Ribecai, A. Microwave-assisted synthesis of indole-derivatives via cycloisomerization of 2-alkynylanilines in water without added catalysts, acids, or bases. Tetrahedron Lett. 2009, 50, 6877–6881. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Parisi, L.M. 2-Aryl and 2-Heteroaryl Indoles from 1-Alkynes and o -Iodotrifluoroacetanilide through a Domino Copper-Catalyzed Coupling−Cyclization Process. Org. Lett. 2003, 5, 3843–3846. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Z.; Zhao, W.; Wei, H.X.; Dufour, M.; Farina, V.; Senanayake, C.H. A practical mild, one-pot, regiospecific synthesis of 2,3-disubstituted indoles via consecutive Sonogashira and Cacchi reactions. Org. Lett. 2006, 8, 3271–3274. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Ouyang, L.; Li, C.; Wu, W.; Jiang, H. N-Heterocyclic carbene palladium-catalyzed cascade annulation/alkynylation of 2-alkynylanilines with terminal alkynes. Org. Biomol. Chem. 2017, 15, 7898–7908. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Z.; Zhang, F.; Xie, S.; Li, H.; Li, P.; Zhou, X. Palladium nanoparticles confined in the cages of MIL-101: An efficient catalyst for the one-pot indole synthesis in water. ACS Catal. 2011, 1, 1604–1612. [Google Scholar] [CrossRef]
- Song, S.; Huang, M.; Li, W.; Zhu, X.; Wan, Y. Efficient synthesis of indoles from 2-alkynylaniline derivatives in water using a recyclable copper catalyst system. Tetrahedron 2015, 71, 451–456. [Google Scholar] [CrossRef]
- Sun, H.; Xiao, L.; Li, W.; Xie, Q.; Shao, L. On Water Silver(I)-Catalyzed Cycloisomerization of Acetylenic Free Amine/Amide towards 7-Azaindole/Indoles/Isochinolone Derivates. Synthesis 2017, 49, 4845–4852. [Google Scholar] [CrossRef] [Green Version]
- Lippincott, D.J.; Landstrom, E.; Cortes-Clerget, M.; Lipshutz, B.H.; Buescher, K.; Schreiber, R.; Durano, C.; Parmentier, M.; Ye, N.; Wu, B.; et al. Surfactant Technology: With New Rules, Designing New Sequences Is Required! Org. Process Res. Dev. 2020, 24, 841–849. [Google Scholar] [CrossRef]
- Anisari, T.N.; Jasinski, J.B.; Leahy, D.K.; Handa, S. Metal–Micelle Cooperativity: Phosphine Ligand-Free Ultrasmall Palladium(II) Nanoparticles for Oxidative Mizoroki–Heck-type Couplings in Water at Room Temperature. JACS Au 2021, 1, 308–315. [Google Scholar] [CrossRef]
- Pang, H.; Hu, Y.; Gallou, F.; Lipshutz, B. Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki–Heck Couplings under Aqueous Micellar Catalysis Conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382. [Google Scholar] [CrossRef]
- Lipshutz, B.H. Synthetic chemistry in a water world. New rules ripe for discovery. Curr. Opin. Green Sustain. Chem. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Landstrom, E.B.; Akporji, N.; Lee, N.R.; Gabriel, C.M.; Braga, F.C.; Lipshutz, B.H. One-Pot Synthesis of Indoles and Pyrazoles via Pd-Catalyzed Couplings/Cyclizations Enabled by Aqueous Micellar Catalysis. Org. Lett. 2020, 22, 6543–6546. [Google Scholar] [CrossRef] [PubMed]
- Risi, C.; Cini, E.; Petricci, E.; Saponaro, S.; Taddei, M. In water Markovnikov hydration and one-pot reductive hydroamination of terminal alkynes under Ruthenium nanoparticle catalysis. Eur. J. Inorg. Chem. 2020, 2020, 1000–1003. [Google Scholar] [CrossRef]
- Risi, C.; Calamante, M.; Cini, E.; Faltoni, V.; Petricci, E.; Rosati, F.; Taddei, M. In water alkylation of amines with alcohols through a borrowing hydrogen process catalysed by ruthenium nanoparticles. Green Chem. 2020, 22, 327–331. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Abela, A.R.; Moser, R.; Nishikata, T.; Duplais, C.; Krasovskiy, A.; Gaston, R.D.; Gadwood, R.C. TPGS-750-M: A Second-Generation Amphiphile for Metal-Catalyzed Cross-Couplings in Water at Room Temperature. J. Org. Chem. 2011, 76, 4379–4391. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.P.; Gallou, F.; Klumphu, P.; Takale, B.S.; Lipshutz, B.H. Structure of Nanoparticles Derived from Designer Surfactant TPGS-750-M in Water, As Used in Organic Synthesis. Chem. A Eur. J. 2018, 24, 6778–6786. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Ghorai, S.; Cortes-Clerget, M. The Hydrophobic Effect Applied to Organic Synthesis: Recent Synthetic Chemistry “in Water”. Chem. A Eur. J. 2018, 24, 6672–6695. [Google Scholar] [CrossRef]
- Alacid, E.; Alonso, D.A.; Botella, L.; Nájera, C.; Pacheco, M.C. Oxime palladacycles revisited: Stone-stable complexes nonetheless very active catalysts. Chem. Rec. 2006, 6, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Petricci, E.; Risi, C.; Ferlin, F.; Lanari, D.; Vaccaro, L. Avoiding hot-spots in Microwave-Assisted Pd/C catalysed reactions by using the biomass derived solvent γ-Valerolactone. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Feng, Y.; Li, F.; Han, J.; He, Y.; Fan, Q.H. A Synthetic Route to Chiral Benzo-Fused N-Heterocycle via Sequential Intramolecular Hydroammination and Asymmetric Hydrogenation of Anilino-Alkynes. Organometallics 2019, 38, 3979–3990. [Google Scholar] [CrossRef]
- Gazvoda, M.; Virant, M.; Pinter, B.; Košmrlj, J. Mechanism of copper-free Sonogashira reaction operates through palladium-palladium transmetallation. Nat. Commun. 2018, 9, 4814. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Song, Q. Copper-Catalyzed Radical Difluoroalkylation and Redox Annulation of Nitroalkynes for the Costruction of C2-Tetrasustituted Indolin-3-Ones. Org. Lett. 2018, 20, 393–396. [Google Scholar] [CrossRef]
- Krause, N. New surfactants for chemistry in water. Curr. Opin. Green Sustain. Chem. 2017, 7, 18–22. [Google Scholar] [CrossRef]
- Fang, Y.Q.; Lautens, M. A Highly Selective Tandem Cross-Coupling of Gem-Dihaloolefins for a Modular, Efficient, Synthesis of Highly Functionalyzed Indoles. J. Org. Chem. 2008, 73, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Maizuru, N.; Inami, T.; Kurahashi, T.; Matsubara, S. Nickel -Catalyzed Cycloaddition of Anthranilic Acid Derivates to Alkynes. Org. Lett. 2011, 13, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fu, H.; Qiao, R.; Jiang, Y.; Zhao, Y. Palladium-Free Copper -Catalyzed Sonogashira Cross-Coupling at Room Temperature. Synthesis 2008, 15, 2417–2426. [Google Scholar] [CrossRef]
- Barluenga, J.; Fenàndez, M.A.; Aznar, F.; Valdés, C. Cascade Alkenyl Amination/Heck Reaction Promoted by a Bifuctional Palladium Catalyst: A Novel One-Pot Synthesis of Indoles from o-Haloaniline and Alkenyl Halides. Chem. Eur. J. 2005, 11, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, I.; Cacchi, S.; Fabrizi, G. Palladium-Catalyzed Synthesis of 2-(Aminomethyl)indoles from Ethyl 3-(o-Trifluoroacetamidophenyl)-1- propargyl Carbonate. Org. Lett. 2006, 8, 2083–2086. [Google Scholar] [CrossRef] [PubMed]



| Ent. | Metal Salts/Ligand 1 | Additive | Type of Heating | Time (min)/ Temperature (T°) 2 | Yield (2) | (1) Recovered |
|---|---|---|---|---|---|---|
| 1 | Pd(PPh3)2Cl2 (10 mol%)/XPhos (10 mol%) | -- | MW | 100 °C, 30 min 3 | 0% | 80% |
| 2 | Pd(MeCN)2Cl2 (10 mol%)/XPhos (10 mol) | -- | MW | 100 °C, 30 min, then 150 °C 30 min 3 | 25% | 60% |
| 3 | Pd(OAc)2 (10 mol%)/Xphos (10 mol%) | -- | MW | 100 °C, 30 min 3 | 25% | 63% |
| 4 | Pd(OAc)2 (10 mol%)/Xphos (10 mol%) | -- | MW | 80 °C, 20 +10 min 3 | 25% | 55% |
| 5 | Pd(OAc)2 (10 mol%)/PPh3 (10 mol%) | -- | MW | 80 °C, 20 +10 min 3 | 0% | 82% |
| 6 | Pd-Oxime (10 mol) | -- | MW | 80 °C, 3 × 20 min 3 | 45% | 30% |
| 7 | Pd(OAc)2 (10 mol%) | MW | 80 °C, 3 × 20 min 3 | 35% | 40% | |
| 8 | Pd(OAc)2 (10 mol%) | -- | MW | 80 °C, 3 × 40 min 3 | 45% | 25% |
| 9 | Pd(OAc)2 (10 mol%) | -- | Oil bath | 80 °C, 16 h | 35% | 30% |
| 10 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | MW | 80 °C, 3 × 20 min 3 | 50% | 10% |
| 11 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | MW | 100 °C. 10 min | 70% | -- |
| 12 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | Oil bath | 100 °C, 4 h | 67% | -- |
| 13 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | Oil bath | 100 °C, 8 h | 30% | -- |
| 14 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | Oil bath | 80 °C, 6 h | 76% | -- |
| 15 | Pd(OAc)2 (10 mol%) | TFA (1 eq) | MW | 100 °C, 10 min | 53% | -- |
| 16 | Pd(OAc)2 (10 mol%) | C11H23COOH (15 mol%) | MW | 100 °C, 10 min | 40% | 35% |
| 17 | Pd(OAc)2 (10 mol%) | DPBA (15 mol%) | MW | 100 °C, 10 min | 45% | 30% |
| 18 | Pd(OAc)2 (5 mol%) | AcOH (1 eq)) | MW | 100 °C, 10 min 4 | 72% | -- |
| 19 | Pd(OAc)2 (2 mol%) | AcOH (1 eq) | MW | 100 °C, 10 min 5 | 43% | 39% |
| 20 | Pd(OAc)2 (10 mol) | AcOH (1 eq) | MW | 100 °C, 10 min 6 | 37% | 40% |
| 21 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | MW | 100 °C, 10 min 7 | 48% | 35% |
| 22 | Pd(OAc)2 (10 mol%) | AcOH (1 eq) | MW | 100 °C, 10 min 8 | 45% | 35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, S.; Cini, E.; Taddei, M.; Vinciarelli, G. Synthesis of 2-Substitued Indoles via Pd-Catalysed Cyclization in an Aqueous Micellar Medium. Molecules 2021, 26, 3917. https://doi.org/10.3390/molecules26133917
Siciliano S, Cini E, Taddei M, Vinciarelli G. Synthesis of 2-Substitued Indoles via Pd-Catalysed Cyclization in an Aqueous Micellar Medium. Molecules. 2021; 26(13):3917. https://doi.org/10.3390/molecules26133917
Chicago/Turabian StyleSiciliano, Sofia, Elena Cini, Maurizio Taddei, and Giorgia Vinciarelli. 2021. "Synthesis of 2-Substitued Indoles via Pd-Catalysed Cyclization in an Aqueous Micellar Medium" Molecules 26, no. 13: 3917. https://doi.org/10.3390/molecules26133917