Development of UHPLC/Q-TOF Analysis Method to Screen Glycerin for Direct Detection of Process Contaminants 3-Monochloropropane-1,2-diol Esters (3-MCPDEs) and Glycidyl Esters (GEs)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calibration Curves
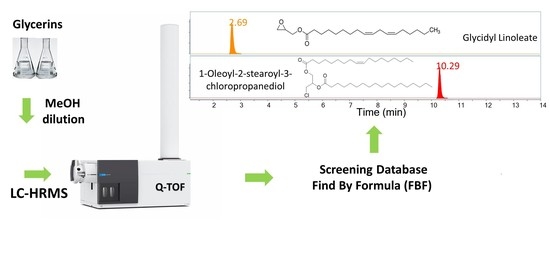
2.2. Analysis of Glycerin Samples
3. Materials and Methods
3.1. Materials
3.1.1. Instrumentation
3.1.2. Chemicals
3.1.3. Glycerin Samples
3.2. Preparation of Stock Solutions and Working Standards
3.3. Preparation of a Calibration Curve of the Mixed Standard in Glycerin and Methanol
3.4. Glycerin Sample Preparation for Screening for MCPDEs and GEs
3.5. UHPLC Analysis
3.6. HRMS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FDA. Food and Drug Administration Investigates Animal Illnesses Linked to Jerky Pet Treats. 2018. Available online: https://www.fda.gov/AnimalVeterinary/NewsEvents/ucm360951.htm (accessed on 12 March 2020).
- FDA. Jerky Pet Treat Investigation Testing Rationale and Results for October 1, 2013–December 31, 2015. 2016. Available online: https://www.fda.gov/media/97687/download (accessed on 15 May 2020).
- Carmichael, N.; Lee, J.; Giger, U. Fanconi syndrome in dog in the UK. Vet. Rec. 2014, 174, 357–358. [Google Scholar] [CrossRef]
- Abraham, L.A.; Tyrrell, D.; Charles, J.A. Transient renal tubulopathy in a racing Greyhound. Aust. Vet. J. 2006, 84, 398–401. [Google Scholar] [CrossRef]
- Thompson, M.F.; Fleeman, L.M.; Kessell, A.E.; Steenhard, L.A.; Foster, S.F. Acquired proximal renal tubulopathy in dogs exposed to a common dried chicken treat: Retrospective study of 108 cases (2007–2009). Aust. Vet. J. 2013, 91, 368–373. [Google Scholar] [CrossRef]
- Hooper, A.N.; Roberts, B.K. Fanconi syndrome in four non-basenji dogs exposed to chicken jerky treats. J. Am. Anim. Hosp. Assoc. 2011, 47, e178–e187. [Google Scholar] [CrossRef]
- Herath, K.; Girard, L.; Reimschuessel, R.; Jayasuriya, H. Application of time-of-flight mass spectrometry for screening of crude glycerins for toxic phorbol ester contaminants. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1046, 226–234. [Google Scholar] [CrossRef]
- Hrncirik, K.; van Duijn, G. An initial study on the formation of 3-MCPD esters during oil refining. Eur. J. Lipid Sci. Technol. 2011, 113, 374–379. [Google Scholar] [CrossRef]
- Arisseto, A.P.; Marcolino, P.F.; Vicente, E. 3-Monochloropropane-1,2-diol fatty acid esters in commercial deep-fat fried foods. Food Addit. Contam. Part A 2015, 32, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Mogol, B.A.; Pye, C.; Anderson, W.; Crews, C.; Gokmen, V. Formation of monochloropropane-1,2-diol and its esters in biscuits during baking. J. Agric. Food Chem. 2014, 62, 7297–7301. [Google Scholar] [CrossRef]
- Hamlet, C.G.; Sadd, P.A.; Crews, C.; Velisek, J.; Baxter, D.E. Occurrence of 3-chloro-propane-1,2-diol (3-MCPD) and related compounds in foods: A review. Food Addit. Contam. 2002, 19, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Baer, I.B.; Calle, B.D.L.; Taylor, P. 3-MCPD in food other than soy sauce or hydrolysed vegetable protein (HVP). Anal. Bioanal. Chem. 2010, 396, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Tarres, A.; Goldmann, T.; Empl, A.M.; Donaubauer, A.; Seefelder, W. Comparison of indirect and direct quantification of esters of monochloropropanediol in vegetable oil. J. Chromatogr. A 2012, 1236, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, L. EFSA Panel on Contaminants in the Food Chain. Risks for human health related to the presence of 3- and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016, 14, e04426. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Update of the risk assessment on 3-monochloropropane diol and its fatty acid esters. EFSA J. 2018, 16, e05083. [Google Scholar] [CrossRef] [PubMed]
- Barocelli, E.; Corradi, A.; Mutti, A.; Petronini, P.G. Comparison between 3-MCPD and its palmitic esters in a 90-day toxicological study. EFSA Support. Publ. 2011, 8, 187E. [Google Scholar] [CrossRef]
- Liu, M.; Gao, B.-Y.; Qin, F.; Wu, P.-P.; Shi, H.-M.; Luo, W.; Ma, A.-N.; Jiang, Y.-R.; Xu, X.-B.; Yu, L.-L. Acute oral toxicity of 3-MCPD mono- and di-palmitic esters in Swiss mice and their cytotoxicity in NRK-52E rat kidney cells. Food Chem. Toxicol. 2012, 50, 3785–3791. [Google Scholar] [CrossRef]
- Abraham, K.; Appel, K.E.; Berger-Preiss, E.; Apel, E.; Gerling, S.; Mielke, H.; Creutzenberg, O.; Lampen, A. Relative oral bioavailability of 3-MCPD from 3-MCPD fatty acid esters in rats. Arch. Toxicol. 2013, 87, 649–659. [Google Scholar] [CrossRef]
- Onami, S.; Cho, Y.M.; Toyoda, T.; Akagi, J.; Fujiwara, S.; Ochiai, R.; Tsujino, K.; Nishikawa, A.; Ogawa, K. Orally administered glycidol and its fatty acid esters as well as 3-MCPD fatty acid esters are metabolized to 3-MCPD in the F344 rat. Regul. Toxicol. Pharmacol. 2015, 73, 726–731. [Google Scholar] [CrossRef]
- Bakhiya, N.; Abraham, K.; Gurtler, R.; Appel, K.E.; Lampen, A. Toxicological assessment of 3-chloropropane-1,2-diol and glycidol fatty acid esters in food. Mol. Nutr. Food Res. 2011, 55, 509–521. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; Abo-Zied, F.S.; Salem, S.T. Effects of subacute 3-monochloropropane-1,2-diol treatment on the kidney of male albino rats. Biotech. Histochem. 2019, 94, 199–203. [Google Scholar] [CrossRef]
- Irwin, R.D.; Eustis, S.L.; Stefanski, S.; Haseman, J.K. Carcinogenicity of glycidol in F344 rats and B6C3F1 mice. J. Appl. Toxicol. 1996, 16, 201–209. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Zhang, Z.; Liu, J.; Wang, Y.L.; Gao, B.; Niu, Y.; Sun, X.; Yu, L. Formation of 3-MCPD Fatty Acid Esters from Monostearoyl Glycerol and the Thermal Stability of 3-MCPD Monoesters. J. Agric. Food Chem. 2016, 64, 8918–8926. [Google Scholar] [CrossRef] [Green Version]
- Mbamalu, V.C. Glycerin and the Market. Master’s Thesis, The University of Tennessee at Chattanooga, Chattanooga, TN, USA, May 2013. [Google Scholar]
- Pudel, F.; Benecke, P.; Fehling, P.; Freudenstein, A.; Matthäus, B.; Schwaf, A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur. J. Lipid Sci. Technol. 2011, 113, 368–379. [Google Scholar] [CrossRef]
- Destaillats, F.; Craft, B.D.; Sandoz, L.; Nagy, K. Formation mechanisms of monochloropropanediol (MCPD) fatty acid diesters in refined palm (Elaeis guineensis) oil and related fractions. Food Addit. Contam. Part A 2012, 29, 29–37. [Google Scholar] [CrossRef]
- Destaillats, F.; Craft, B.D.; Dubois, M.l.; Nagy, K. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part I: Formation mechanism. Food Chem. 2012, 131, 1391–1398. [Google Scholar] [CrossRef]
- MacMahon, S.; Mazzola, E.; Begley, T.H.; Diachenko, G.W. Analysis of processing contaminants in edible oils. Part 1. Liquid chromatography-tandem mass spectrometry method for the direct detection of 3-monochloropropanediol monoesters and glycidyl esters. J. Agric. Food Chem. 2013, 61, 4737–4747. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, S.; Begley, T.H.; Diachenko, G.W. Analysis of processing contaminants in edible oils. Part 2. Liquid chromatography-tandem mass spectrometry method for the direct detection of 3-monochloropropanediol and 2-monochloropropanediol diesters. J. Agric. Food Chem. 2013, 61, 4748–4757. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, S.; Ridge, C.D.; Begley, T.H. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the direct detection of 2-monochloropropanediol (2-MCPD) esters in edible oils. J. Agric. Food Chem. 2014, 62, 11647–11656. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Matsubara, A.; Uchikata, T.; Tsumura, K.; Fukusaki, E.; Bamba, T. High-throughput and sensitive analysis of 3-monochloropropane-1,2-diol fatty acid esters in edible oils by supercritical fluid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1250, 99–104. [Google Scholar] [CrossRef] [PubMed]
- FDA. Acceptance Criteria for Confirmation of Identity of Chemical Residues using Exact Mass Data within the Office of Foods and Veterinary Medicine. 2015. Available online: https://www.fda.gov/media/96499/download (accessed on 20 August 2020).


| Compound | Exact Mass | (M+H) | (M+Na) |
|---|---|---|---|
| Glycidyl linolenate | 334.2508 | 2,209,671 | 34,331,876 |
| Glycidyl linoleate | 336.2664 | 3,308,840 | 45,741,444 |
| 1-Palmitoyl-3-chloropropanediol | 348.2431 | 0 | 1,517,938 |
| 2-Oleoyl-3-chloropropanediol | 374.2588 | 116,159 | 6,265,336 |
| 1-Oleoyl-2-stearoyl-3-chloropropanediol | 640.5197 | 0 | 7,484,321 |
| 1-Palmitoyl-2-stearoyl-3-chloropropanediol | 614.5041 | 0 | 8,217,162 |
| Formula | Exact Mass | Compound Name |
|---|---|---|
| MCPD monoesters | ||
| C17H33ClO3 | 320.2118 | Myristoyl-chloropropanediol |
| C15H29ClO3 | 292.1805 | Lauroyl-chloropropanediol |
| C19H37ClO3 | 348.2431 | Palmitoyl-chloropropanediol |
| C21H41ClO3 | 376.2744 | Stearoyl-chloropropanediol |
| C21H39ClO3 | 374.2588 | Oleyl-chloropropanediol |
| C21H37ClO3 | 372.2431 | Linoleoyl-chloropropanediol |
| C21H35ClO3 | 370.2275 | Linolenoyl-chloropropanediol |
| MCPD diesters | ||
| C35H67ClO4 | 586.4728 | Di-palmitoyl-chloropropanediol |
| C27H51ClO4 | 474.3476 | Di-lauroyl-chloropropanediol |
| C29H55ClO4 | 502.3789 | Lauroyl-myristoyl-chloropropanediol |
| C33H57ClO4 | 552.3945 | Lauroyl-linolenoyl-chloropropanediol |
| C33H59ClO4 | 554.4102 | Lauroyl-linoleoyl-chloropropanediol |
| C39H67ClO4 | 634.4728 | Di-linoleoyl-chloropropanediol |
| C39H63ClO4 | 630.4415 | Di-linolenoyl-chloropropanediol |
| C35H61ClO4 | 580.4285 | Linolenoyl-myristoyl-chloropropanediol |
| C31H59ClO4 | 530.4102 | Di-myristoyl-chloropropanediol |
| C31H59ClO4 | 530.4102 | Lauroyl-Palmitoyl-chloropropanediol |
| C33H61ClO4 | 556.4258 | Lauroyl-Oleoyl-chloropropanediol |
| C39H65ClO4 | 632.4571 | Linoleoyl-Linolenoyl-chloropropanediol |
| C35H63ClO4 | 582.4415 | Myristoyl-Linoleoyl-chloropropanediol |
| C33H63ClO4 | 558.4415 | Myristoyl-palmitoyl-chloropropanediol |
| C33H63ClO4 | 558.4415 | Lauroyl-stearoyl-chloropropanediol |
| C35H65ClO4 | 584.4571 | Myristoyl-oleoyl-chloropropanediol |
| C39H71ClO4 | 638.5041 | Stearoyl-linoleoyl-chloropropanediol |
| C39H73ClO4 | 640.5197 | Oleoyl-stearoyl-chloropropanediol |
| C39H71ClO4 | 638.5041 | Linoleoyl-stearoyl-chloropropanediol |
| C37H71ClO4 | 614.5041 | Palmitoyl-stearoyl-chloropropanediol |
| C37H67ClO4 | 610.4728 | Palmitoyl-linoleoyl-chloropropanediol |
| C37H65ClO4 | 608.4571 | Palmitoyl-linolenoyl-chloropropanediol |
| C35H67ClO4 | 586.4728 | Myristoyl-stearoyl-chloropropanediol |
| C39H71ClO4 | 638.5041 | Di-oleoyl-chloropropanediol |
| C37H69ClO4 | 612.4884 | Oleoyl-palmitoyl-chloropropanediol |
| C39H75ClO4 | 642.5354 | Di-stearoyll-chloropropanediol |
| C39H67ClO4 | 634.4728 | Oleoyl-linolenoyl-chloropropanediol |
| C39H69ClO4 | 636.4884 | Oleoyl-linoleoyl-chloropropanediol |
| C37H69ClO4 | 612.4884 | Oleyl-palmitoyl-chloropropanediol |
| Glycidyl esters (GEs) | ||
| C15H28O3 | 256.2038 | Glycidyl laurate |
| C17H32O3 | 284.2351 | Glycidyl myristate |
| C21H40O3 | 340.2977 | Glycidyl stearate |
| C19H36O3 | 312.2664 | Glycidyl palmitate |
| C21H38O3 | 338.2821 | Glycidyl oleate |
| C21H36O3 | 336.2664 | Glycidyl linoleate |
| C21H34O3 | 334.2508 | Glycidyl linoleate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, L.; Herath, K.; Escobar, H.; Reimschuessel, R.; Ceric, O.; Jayasuriya, H. Development of UHPLC/Q-TOF Analysis Method to Screen Glycerin for Direct Detection of Process Contaminants 3-Monochloropropane-1,2-diol Esters (3-MCPDEs) and Glycidyl Esters (GEs). Molecules 2021, 26, 2449. https://doi.org/10.3390/molecules26092449
Girard L, Herath K, Escobar H, Reimschuessel R, Ceric O, Jayasuriya H. Development of UHPLC/Q-TOF Analysis Method to Screen Glycerin for Direct Detection of Process Contaminants 3-Monochloropropane-1,2-diol Esters (3-MCPDEs) and Glycidyl Esters (GEs). Molecules. 2021; 26(9):2449. https://doi.org/10.3390/molecules26092449
Chicago/Turabian StyleGirard, Lauren, Kithsiri Herath, Hernando Escobar, Renate Reimschuessel, Olgica Ceric, and Hiranthi Jayasuriya. 2021. "Development of UHPLC/Q-TOF Analysis Method to Screen Glycerin for Direct Detection of Process Contaminants 3-Monochloropropane-1,2-diol Esters (3-MCPDEs) and Glycidyl Esters (GEs)" Molecules 26, no. 9: 2449. https://doi.org/10.3390/molecules26092449