5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Silico Determination of Lipophilicity as Clog P
2.3. Biological Evaluation
2.4. Molecular Properties Prediction—Lipinski “Rule of Five”
2.5. Computational Studies—Docking Simulation Soybean Lipoxygenase
Molecular Modeling of the Synthesized Derivatives in Soybean LOX
3. Experimental Section
3.1. Materials and Instruments
3.2. Chemistry General Procedure
3.2.1. Synthesis of N-Benzoylglycine Hippuric Acid (1)
3.2.2. Synthesis of (Z)-4-Arylidene-2-phenyloxazol-5(4H)-ones 2a–f
3.2.3. Synthesis of (Z)-Morpholino-benzamides 3a, 3c, 3d
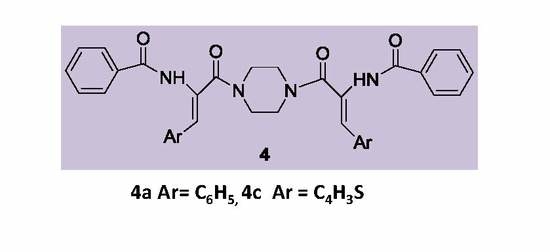
3.2.4. Synthesis of (Z,Z′)-Piperazine-bisbenzamides 4a–4e
3.2.5. Synthesis of (Z)-N-(3-Oxo-3-(piperidin-1-yl)-1-(thiophen-2-yl)prop-1-en-2-yl)benzamide (5c)
3.2.6. Synthesis of (Z)-Ethyl 2-benzamido-3-(thiophen-2-yl)acrylate (6c)
3.3. Biological Assays
3.3.1. Biological In Vitro Assays
Inhibition of Linoleic Acid Lipid Peroxidation
Soybean Lipoxygenase Inhibition Study
Tyrosinase Inhibition Assay
Inhibition of Trypsin Induced Proteolysis
3.3.2. Biological In Vivo Assays
Inhibition of the Carrageenin-Induced Edema
Anti-Nociception—Writhing Test
3.4. Molecular Properties Prediction-Lipinski “Rule of Five”
3.5. Computational Methods. Molecular Docking Studies on Soybean Lipoxygenase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Nat. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W. Eicosanoid nomenclature. Prostaglandins 1989, 38, 125–133. [Google Scholar] [CrossRef]
- Stables, M.J.; Gilroy, D.W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Isakson, P.C.; Seibert, K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Nat. Acad. Sci. USA 1994, 91, 3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Szostak, M. Recent Developments in the Synthesis and Reactivity of Isoxazoles: Metal Catalysis and Beyond. Adv. Synth. Catal. 2015, 357, 2583–2614. [Google Scholar] [CrossRef]
- Morita, T.; Yugandar, S.; Fuse, S.; Nakamura, H. Recent progresses in the synthesis of functionalized isoxazoles. Tetrahedron Lett. 2018, 59, 1159–1171. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Combining Molecular Scaffolds from FDA Approved Drugs: Application to Drug Discovery. J. Med. Chem. 2017, 60, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Goyard, D.; Kónya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; et al. Glucose-derived spiro-isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.-B.; Wityak, J.; Sielecki, T.M.; Pinto, D.J.; Batt, D.G.; Cain, G.A.; Sworin, M.; Rockwell, A.L.; Roderick, J.J.; Wang, S.; et al. Discovery of an Orally Active Series of Isoxazoline Glycoprotein IIb/IIIa Antagonists. J. Med. Chem. 1997, 40, 2064–2084. [Google Scholar] [CrossRef]
- Cheng, K.F.; Al-Abed, Y. Critical modifications of the ISO-1 scaffold improve its potent inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity. Bioorg. Med. Chem. Let. 2006, 16, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- Puig, C.; Crespo, M.I.; Godessart, N.; Feixas, J.; Ibarzo, J.; Jiménez, J.-M.; Soca, L.; Cardelús, I.; Heredia, A.; Miralpeix, M.; et al. Synthesis and Biological Evaluation of 3,4-Diaryloxazolones: A New Class of Orally Active Cyclooxygenase-2 Inhibitors. J. Med. Chem. 2000, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Costa, D.; Toste, S.A.; Lima, J.L.F.C.; Reis, S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic. Biol. Med. 2004, 37, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Talley, J.J.; Brown, D.L.; Carter, J.S.; Graneto, M.J.; Koboldt, C.M.; Masferrer, J.L.; Perkins, W.E.; Rogers, R.S.; Shaffer, A.F.; Zhang, Y.Y.; et al. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, valdecoxib: A potent and selective inhibitor of COX-2. J. Med. Chem. 2000, 43, 775–777. [Google Scholar] [CrossRef]
- Talley, J.J.; Bertenshaw, S.R.; Brown, D.L.; Carter, J.S.; Graneto, M.J.; Kellogg, M.S.; Koboldt, C.M.; Yuan, J.; Zhang, Y.Y.; Seibert, K. N-[[(5-methyl-3-phenylisoxazol-4-yl)-phenyl]sulfonyl]propanamide, sodium salt, parecoxib sodium: A potent and selective inhibitor of COX-2 for parenteral administration. J. Med. Chem. 2000, 43, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Dündar, Y.; Ünlü, S.; Banoğlu, E.; Entrena, A.; Costantino, G.; Nunez, M.-T.; Ledo, F.; Şahin, M.F.; Noyanalpan, N. Synthesis and biological evaluation of 4,5-diphenyloxazolone derivatives on route towards selective COX-2 inhibitors. Eur. J. Med. Chem. 2009, 44, 1830–1837. [Google Scholar]
- Zhang, J.; Ding, E.L.; Song, Y. Adverse Effects of Cyclooxygenase 2 Inhibitors on Renal and Arrhythmia EventsMeta-analysis of Randomized Trials. JAMA 2006, 296, 1619–1632. [Google Scholar] [CrossRef]
- Fozooni, S.; Tikdari, A.M.; Hamidian, H.; Khabazzadeh, H. A synthesis of some new 4-arylidene-5(4H)-oxazolone azo dyes and an evaluation of their solvatochromic behaviour. Arkivoc 2008, 2008, 115–123. [Google Scholar]
- Banerjee, J.; Sharma, N. A review on oxazolone, it’s method of synthesis and biological activity. Eur. J. Biomed. Pharm. Sci. 2015, 2, 964–987. [Google Scholar]
- Towns, A.D. Developments in azo disperse dyes derived from heterocyclic diazo components. Dyes Pigments 1999, 42, 3–28. [Google Scholar] [CrossRef]
- El-Mekabaty, A. Erlenmeyer Azlactones: Synthesis, Reactions and Biological Activity. Int. J. Mod. Org. Chem. 2013, 2, 40–66. [Google Scholar]
- Marra, I.F.S.; de Castro, P.P.; Amarante, G.W. Recent Advances in Azlactone Transformations. Eur. J. Org. Chem. 2019, 2019, 5830–5855. [Google Scholar] [CrossRef]
- Michaelidou, A.S.; Hadjipavlou-Litina, D. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): A Comparative QSAR Study. Chem. Rev. 2005, 105, 3235–3271. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Khan, S.A.; Chawla, G.; Panda, B.P. Synthesis and antimicrobial screening of N-[2-(2/4-substituted phenyl)-1-(5/6 substituted 1H-benzimidazol-2-yl)vinyl] benzamides. Acta Pol. Pharm. 2012, 69, 629–636. [Google Scholar] [PubMed]
- Barros, T.G.; Pinheiro, S.; Williamson, J.S.; Tanuri, A.; Gomes, M.; Pereira, H.S.; Brindeiro, R.M.; Neto, J.B.A.; Antunes, O.A.C.; Muri, E.M.F. Pseudo-peptides derived from isomannide: Inhibitors of serine proteases. Amino Acids 2010, 38, 701–709. [Google Scholar] [CrossRef]
- Boulos, L.S.; Ewies, E.F.; Fahmy, A.F.M. Synthesis of New Bisphosphonate and Bisphosphonic Acid Derivatives and Heterocyclic and Dialkylcarbamoyl Oxazolone Derivatives with Anticancer and Antischistosomal Activity. Z. Naturforsch. B 2011, 66, 1056–1068. [Google Scholar]
- Girgis, A.S.; Ellithey, M. Facile synthesis of non-steroidal anti-inflammatory active bisbenzamide-containing compounds. Bioorg. Med. Chem. 2006, 14, 8527–8532. [Google Scholar] [CrossRef]
- Polgár, L. The catalytic triad of serine peptidases. Cell. Mol. Life Sci. 2005, 62, 2161–2172. [Google Scholar] [CrossRef]
- Pinto, I.L.; West, A.; Debouck, C.M.; DiLella, A.G.; Gorniak, J.G.; O’Donnell, K.C.; O’Shannessy, D.J.; Patel, A.; Jarvest, R.L. Novel, selective mechanism-based inhibitors of the herpes proteases. Bioorg. Med. Chem. Lett. 1996, 6, 2467–2472. [Google Scholar] [CrossRef]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg. Med. Chem. Lett. 2004, 14, 611–614. [Google Scholar] [CrossRef]
- Rao, Y.S. Reactions in polyphosphoric acid. I. New stereospecific synthesis of the E isomers of 2-phenyl-4-arylmethylene-2-oxazolin-5-ones. J. Org. Chem. 1976, 41, 722–725. [Google Scholar] [CrossRef]
- Valdés, R.; Aranda, D.; Alvarez, H.; Antunes, O. Microwave-Promoted Ring Opening Reaction of Azalactones. Lett. Org. Chem. 2007, 4, 35–38. [Google Scholar] [CrossRef]
- Gibbs, R.J.; Timasheff, S.N.; Nord, F.F. Aminolysis and Alcoholysis of a Thiophene Azlactone. J. Am. Chem. Soc. 1951, 73, 5877–5878. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, K.; Gao, M.; Jiang, Z.; Liu, J.; Ma, Y.; Wang, H.; Tan, Q.; Xiao, J.; Xu, B. Copper nitrate-mediated synthesis of 3-aryl isoxazolines and isoxazoles from olefinic azlactones. Org. Biomol. Chem. 2019, 17, 5509–5513. [Google Scholar] [CrossRef] [PubMed]
- Khadse, S.C.; Talele, G.S.; Agrawal, S.S. Aminocarbonyl Arylvinylbenzamides as Gastric Sparing Anti-inflammatory Agents. Arch. Pharm. 2011, 344, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Sarkate, A.P.; Farooqui, M.; Shinde, D.B. Greener approach: Ionic liquid [Et3NH][HSO4]-catalyzed multicomponent synthesis of 4-arylidene-2-phenyl-5(4H)oxazolones under solvent-free condition. Synth. Commun. 2017, 47, 1676–1683. [Google Scholar] [CrossRef]
- Kitazawa, M.; Higuchi, R.; Takahashi, M.; Wada, T.; Sasabe, H. Novel Molecular Design for Second-Harmonic Generation: Azlactone Derivatives. J. Phys. Chem. 1995, 99, 14784–14792. [Google Scholar] [CrossRef]
- Conway, P.A.; Devine, K.; Paradisi, F. A simple and efficient method for the synthesis of Erlenmeyer azlactones. Tetrahedron 2009, 65, 2935–2938. [Google Scholar] [CrossRef]
- Rostami, M.; Khosropour, A.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I. Organic–inorganic hybrid polyoxometalates: Efficient, heterogeneous and reusable catalysts for solvent-free synthesis of azlactones. Appl. Catal. A Gen. 2011, 397, 27–34. [Google Scholar] [CrossRef]
- Trost, B.M.; Morris, P.J.; Sprague, S.J. Palladium-Catalyzed Diastereo- and Enantioselective Formal [3 + 2]-Cycloadditions of Substituted Vinylcyclopropanes. J. Am. Chem. Soc. 2012, 134, 17823–17831. [Google Scholar] [CrossRef] [Green Version]
- Cleary, T.; Brice, J.; Kennedy, N.; Chavez, F. One-pot process to Z-α-benzoylamino-acrylic acid methyl esters via potassium phosphate-catalyzed Erlenmeyer reaction. Tetrahedron Lett. 2010, 51, 625–628. [Google Scholar] [CrossRef]
- Crowe, B.F.; Nord, F.F. Studies on the chemistry of heterocyclics. XI. Further recations on the aldehydes. J. Org. Chem. 1950, 15, 1177–1183. [Google Scholar] [CrossRef]
- Lalitha, K.; Iyengar, D.S.; Bhalerao, U.T. Cyclopropanation of 2-ylideneoxazol-5-one with diphenyldiazomethane. Stereospecific synthesis of novel gem-diphenylcyclopropyl amino acid derivatives. J. Org. Chem. 1989, 54, 1771–1773. [Google Scholar] [CrossRef]
- Lalitha, N.; Bhalerao, U.T.; Iyengar, D.S. Sequential addition of 2-potassio-2-nitropropane and oxygen to 4-arylidene-oxazol-5-ones: A new method for 2-aryl butenoic acid imides. J. Chem. Soc. Chem. Commun. 1991, 13, 897–899. [Google Scholar] [CrossRef]
- Biobyte. Available online: http://www.biobyte.com/ (accessed on 25 June 2020).
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Müller, K. 5-Lipoxygenase and 12-Lipoxygenase: Attractive Targets for the Development of Novel Antipsoriatic Drugs. 5-Lipoxygenase und 12-Lipoxygenase: Attraktive Target-Enzyme für die Entwicklung neuer Antipsoriatika. Arch. Pharm. 1994, 327, 1–19. [Google Scholar]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic determination of the active site iron and its ligands in soybean lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef]
- Skrzypczak-Jankun, E.; Amzel, L.M.; Kroa, B.A.; Funk, M.O., Jr. Structure of soybean lipoxygenase L3 and a comparison with its L1 isoenzyme. Proteins 1997, 29, 15–31. [Google Scholar] [CrossRef]
- Khan, K.M.; Mughal, U.R.; Khan, M.T.H.; Zia, U.; Perveen, S.; Iqbal Choudhary, M. Oxazolones: New tyrosinase inhibitors; synthesis and their structure–activity relationships. Bioorg. Med. Chem. 2006, 14, 6027–6033. [Google Scholar] [CrossRef]
- Rescigno, A.; Sollai, F.; Pisu, B.; Rinaldi, A.; Sanjust, E. Tyrosinase Inhibition: General and Applied Aspects. J. Enzym. Inhib. Med. Chem. 2002, 17, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Canavan, N. FDA and drug companies alike want ADME-tox testing performed earlier and earlier in a drug’s life cycle. Drug Discov. Dev. 2007, 10, 34–36. [Google Scholar]
- Molinspiration Cheminformatics. Available online: www.molinspiration.com (accessed on 1 June 2020).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies. Molecules 2018, 24, 12. [Google Scholar] [CrossRef] [Green Version]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional cinnamic acid derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, I.; Hadjipavlou-Litina, D.; Bilia, A.-R.; Karioti, A. LC-MS- and NMR-Guided Isolation of Monoterpene Dimers from Cultivated Thymus vulgaris Varico 3 Hybrid and Their Antityrosinase Activity. Planta Med. 2019, 85, 941–946. [Google Scholar]
- Kouzi, O.; Pontiki, E.; Hadjipavlou-Litina, D. 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules 2019, 24, 4411. [Google Scholar] [CrossRef] [Green Version]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic Acid for Analgesic Screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget Molecular Hybrids of Cinnamic Acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiser, A.; Sali, A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzym. 2003, 374, 461–491. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |






| Compd. | Clog P * | AAPH% 100 µM | LOX IC50 (μM) or % 100 μM | ΤyrI% 100 μM | IC50 (μM) or Iptr% 10 µM |
|---|---|---|---|---|---|
| 2a | 3.70 | 95 | no | no | 33 |
| 2b | 6.25 | 79 | no | no | 8.25 μM |
| 2c | 3.34 | 91 | no | 16 | 10 μM |
| 2d | 4.86 | 81 | 49 | 5 | 7 μM |
| 2e | 2.87 | 60 | 15 | 3 | 60 μM |
| 2f | 5.80 | no | no | nt | nt |
| 3a | 2.82 | 69 | 100 μM | nt | no |
| 3c | 2.47 | 93 | 39 | 7 | 6.75 μM |
| 3d | 3.99 | 91 | 37 | nt | nt |
| 4a | 6.03 | 82 | 36 | 33 | 8 μM |
| 4b | 11.13 | 99 | 85 μM | 12 | 9 μM |
| 4c | 5.33 | 93 | 41 μM | 42 | 9.1 μM |
| 4d | 8.38 | 90 | 65 μM | 28 | 8.5 μM |
| 4e | 4.39 | 83 | 88 μM | 17 | 8.75 μM |
| 5c | 3.60 | 72 | 41 | 13 | 6.7 μM |
| 6c | 3.21 | 59 | 36 | 5 | 1 μM |
| NDGA | - | 0.45 μM, 93 | - | - | |
| Trolox | 93 | - | - | - | |
| Kojic acid | - | - | IC50 = 2.81 μM | - | |
| Salicylic Acid | 100 μM |
| Compd. | CPE (%) a | Writhing Inhibition (%) a |
|---|---|---|
| 4a | 44 * | 32 |
| 4c | 56 ** | 58 |
| Indomethacin | 58 ** | - |
| Aspirin | - | 77 |
| Compd. | milogP a | TPSA b | No Atoms | NoO,N c | No OH, NH d | No Violations | No Rotational Bonds e | Volume f | MW g |
|---|---|---|---|---|---|---|---|---|---|
| 4a | 5.43 | 98.81 | 44 | 8 | 2 | 2 | 8 | 535.77 | 584.68 |
| 4b | 9.17 | 117.28 | 63 | 10 | 2 | 2 | 15 | 782.76 | 954.72 |
| 4c | 4.87 | 98.81 | 42 | 8 | 2 | 1 | 8 | 517.20 | 596.73 |
| 4d | 7.47 | 98.81 | 53 | 8 | 2 | 2 | 9 | 640.59 | 684.80 |
| 4e | 3.47 | 125.09 | 43 | 10 | 2 | 1 | 9 | 515.74 | 564.60 |
| 5c | 3.57 | 49.41 | 24 | 4 | 1 | 0 | 4 | 309.90 | 340.45 |
| 6c | 3.55 | 55.40 | 21 | 4 | 1 | 0 | 6 | 266.30 | 301.36 |
| Compd. | Method | Time (min) | Yield (%) | Compd. | Method | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 2a | A | 120 | 67 | 3d | E | 15 | 60 |
| 2a | B | 15 | 56 | 4a | F | 75 | 12 |
| 2b | A | 120 | 83 | 4a | G | 10 | 94 |
| 2c | A | 120 | 37 | 4a | H | 4440 | 14 |
| 2c | B | 15 | 66 | 4b | F | 240 | 31 |
| 2c | C | 20 | 19 | 4c | F | 135 | 14 |
| 2d | A | 120 | 53 | 4c | G | 15 | 19 |
| 2d | B | 15 | 38 | 4c | I | 5 | trace |
| 2e | A | 120 | 36 | 4d | F | 90 | 10 |
| 2f | A | 120 | 12 | 4d | G | 20 | 92 |
| 3a | E | 15 | 68 | 4d | I | 5 | trace |
| 3c | D | 120 | 26 | 4e | F | 120 | 54 |
| Compd. | AAPH% 100 µM | LOX IC50 (μM) or % 100 μM | ΤyrI% 100 μM | IC50 (μM) or Iptr% 10 µM | CPE (%) | Writhing Inhibition (%) |
|---|---|---|---|---|---|---|
| 3c | 93 | 39 | 7 | 6.75 μM | - | - |
| 4a | 82 | 36 | 33 | 8 μM | 44 | 32 |
| 4b | 99 | 85 μM | 12 | 9 μM | - | - |
| 4c | 93 | 41 μM | 42 | 9.1 μM | 56 | 58 |
| 4d | 90 | 65 μM | 28 | 8.5 μM | - | - |
| 4e | 83 | 88 μM | 17 | 8.75 μM | - | - |
| 5c | 72 | 41 | 13 | 6.7 μM | - | - |
| 6c | 59 | 36 | 5 | 1 μM | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavridis, E.; Bermperoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D. 5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules. Molecules 2020, 25, 3173. https://doi.org/10.3390/molecules25143173
Mavridis E, Bermperoglou E, Pontiki E, Hadjipavlou-Litina D. 5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules. Molecules. 2020; 25(14):3173. https://doi.org/10.3390/molecules25143173
Chicago/Turabian StyleMavridis, Evangelos, Eleftherios Bermperoglou, Eleni Pontiki, and Dimitra Hadjipavlou-Litina. 2020. "5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules" Molecules 25, no. 14: 3173. https://doi.org/10.3390/molecules25143173