Tocopheryl Succinate-Induced Structural Changes in DPPC Liposomes: DSC and ANS Fluorescence Studies
Abstract
:1. Introduction
2. Results
2.1. Temperature Studies of ANS Fluorescence in DPPC Liposomes with Embedded TS
2.2. Kinetics of ANS Adsorption on DPPC in the Presence of Tocopherol Derivative
2.3. Temperature Dependencies of the ANS Emission Maxima (λmax)
2.4. Fluorescence Lifetime of ANS in DPPC
2.5. ANS Parameters Calculations
2.6. Zeta Potential Measurements
2.7. DSC Measurements
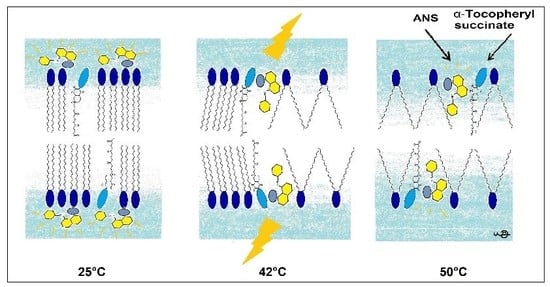
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Vesicles
4.3. Spectroscopic Measurements
4.4. Differential Scanning Calorimetry (DSC)
4.5. Fluorescence Lifetimes
4.6. Fitting Procedures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Barbas, C. Vitamin E: Action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Miyataka, H.; Yamamoto, K.; Hirano, T. Synthesis and Physiological Activity of Novel Tocopheryl Glycosides. Chem. Pharm. Bull. 2001, 49, 948–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, J.B.; She, H.S.; Pownall, H.J. Interaction of vitamin E with saturated phospholipid bilayers. Biochem. Biophys. Res. Commun. 1982, 106, 842–847. [Google Scholar] [CrossRef]
- Massey, J.B. Interfacial properties of phosphatidylcholine bilayers containing vitamin E derivatives. Chem. Phys. Lipids 2001, 109, 157–174. [Google Scholar] [CrossRef]
- Birringer, M.; EyTina, J.H.; Salvatore, B.A.; Neuzil, J. Vitamin E analogues as inducers of apoptosis: Structure–Function relation. Br. J. Cancer 2003, 88, 1948–1955. [Google Scholar] [CrossRef] [Green Version]
- Kogure, K.; Manabe, S.; Suzuki, I.; Tokumura, A.; Fukuzawa, K. Cytotoxicity of alpha-Tocopheryl succinate, malonate and oxalate in normal and cancer cells in vitro and their anti-Cancer effects on mouse melanoma in vivo. J. Nutr. Sci. Vitaminol. 2005, 51, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.N.; Kumar, B.; Yan, X.-D.; Hanson, A.J.; Cole, W.C. α-Tocopheryl Succinate, the Most Effective Form of Vitamin E for Adjuvant Cancer Treatment: A Review. J. Am. Coll. Nutr. 2003, 22, 108–117. [Google Scholar] [CrossRef]
- Kanai, K.; Kikuchi, E.; Mikami, S.; Suzuki, E.; Uchida, Y.; Kodaira, K.; Miyajima, A.; Ohigashi, T.; Nakashima, J.; Oya, M. Vitamin E succinate induced apoptosis and enhanced chemosensitivity to paclitaxel in human bladder cancer cells in vitro and in vivo. Cancer Sci. 2010, 101, 216–223. [Google Scholar] [CrossRef]
- Donapaty, S.; Louis, S.; Horvath, E.; Kun, J.; Sebti, S.M.; Malafa, M.P. RRR-Alpha-Tocopherol succinate down-Regulates oncogenic Ras signaling. Mol. Cancer Ther. 2006, 5, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Malafa, M.P.; Neitzel, L.T. Vitamin E Succinate Promotes Breast Cancer Tumor Dormancy. J. Surg. Res. 2000, 93, 163–170. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Yu, W.; Hou, S.; Zhao, Y.; Zhang, Z.; Huang, X.; Wu, K. Alpha-Tocopheryl succinate enhances doxorubicin-induced apoptosis in human gastric cancer cells via promotion of doxorubicin influx and suppression of doxorubicin efflux. Cancer Lett. 2011, 307, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Mussi, S.V.; Gomes, D.A.; Yoshida, M.I.; Frézard, F.; Carregal, V.; Ferreira, L.A. α-Tocopherol succinate improves encapsulation and anticancer activity of doxorubicin loaded in solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 140, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, Š.; Turánek-Knötigová, P.; Mašek, J.; Korvasová, Z.; Škrabalová, M.; Plocková, J.; Bartheldyová, E.; Turánek, J. Liposomes with high encapsulation capacity for paclitaxel: Preparation, characterisation and in vivo anticancer effect. J. Pharm. Sci. 2010, 99, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, S.; Knotigova, P.T.; Mašek, J.; Prochazka, L.; Lukáč, R.; Miller, A.; Neuzil, J.; Turánek, J. Liposomal delivery systems for anti-Cancer analogues of vitamin E. J. Control. Release 2015, 207, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Phadke, R.S.; Govil, G. Magnetic resonance studies on the binding of etomidate to lipid bilayers. Physiol. Chem. Phys. Med. NMR 1987, 19, 241–250. [Google Scholar]
- Krilov, D.; Kosović, M.; Serec, K. Spectroscopic studies of alpha tocopherol interaction with a model liposome and its influence on oxidation dynamics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 588–593. [Google Scholar] [CrossRef]
- Mason, J.T. Properties of phosphatidylcholine bilayers as revealed by mixed-Acyl phospholipid fluorescent probes containing n-(9-anthroyloxy) fatty acids. Biochim. Biophys. Acta (BBA)—Biomembr. 1994, 1194, 99–108. [Google Scholar] [CrossRef]
- Kogure, K.; Hama, S.; Kisaki, M.; Takemasa, H.; Tokumura, A.; Suzuki, I.; Fukuzawa, K. Structural characteristic of terminal dicarboxylic moiety required for apoptogenic activity of α-Tocopheryl esters. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2004, 1672, 93–99. [Google Scholar] [CrossRef]
- Quinn, P.J. Characterisation of Clusters of alpha-Tocopherol in Gel and Fluid Phases of Dipalmitoylglycerophosphocholine. JBIC J. Biol. Inorg. Chem. 1995, 233, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-Z.; Duzgunes, N.; Szoka, F.C. Effects of replacement of the hydroxyl group of cholesterol and tocopherol on the thermotropic behavior of phospholipid membranes. Biochemistry 1985, 24, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Yamada, T.; Kimura, M.; Oka, T.; Ohshima, H.; Kondo, T. Temperature-and ionic strength-Induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Biophys. Chem. 1991, 41, 175–183. [Google Scholar] [CrossRef]
- Slavík, J. Anilinonaphthalene sulfonate as a probe of membrane composition and function. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 1982, 694, 1–25. [Google Scholar] [CrossRef]
- Gasymov, O.K.; Abduragimov, A.R.; Glasgow, B.J. Molten globule state of tear lipocalin: ANS binding restores tertiary interactions. Biochem. Biophys. Res. Commun. 2007, 357, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Haynes, D.H.; Staerk, H. 1-Anilino-8-naphthalenesulfonate: A fluorescent probe of membrane surface structure, composition and mobility. J. Membr. Biol. 1974, 17, 313–340. [Google Scholar] [CrossRef]
- Bisby, R.H.; Birch, D.J. A time-Resolved fluorescence anisotropy study of bilayer membranes containing alpha-Tocopherol. Biochem. Biophys. Res. Commun. 1989, 158, 386–391. [Google Scholar] [CrossRef]
- De Almeida, R.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Lipid Rafts have Different Sizes Depending on Membrane Composition: A Time-Resolved Fluorescence Resonance Energy Transfer Study. J. Mol. Biol. 2005, 346, 1109–1120. [Google Scholar] [CrossRef]
- Jaśkowski, J.; Witkowski, J.; Myśliwski, A.; Zawadzki, H. Effect of air ions on L 1210 cells: Changes in fluorescence of membrane-Bound 1,8-Aniline-Naphthalene-Sulfonate (ANS) after in vitro exposure of cells to air ions. Gen. Physiol. Biophys. 1986, 5, 511–515. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Partition of membrane probes in a gel/fluid two-component lipid system: A fluorescence resonance energy transfer study. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1467, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, P.; Holowka, D.; Baird, B. Fluorescence Resonance Energy Transfer between Lipid Probes Detects Nanoscopic Heterogeneity in the Plasma Membrane of Live Cells. Biophys. J. 2007, 92, 3564–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vladkova, R.; Teuchner, K.; Leupold, D.; Koynova, R.; Tenchov, B. Detection of the metastable rippled gel phase in hydrated phosphatidylcholine by fluorescence spectroscopy. Biophys. Chem. 2000, 84, 159–166. [Google Scholar] [CrossRef]
- Neuzil, J.; Weber, T.; Gellert, N.; Weber, C. Selective cancer cell killing by α-Tocopheryl succinate. Br. J. Cancer 2001, 84, 87–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentak, D. Alternative methods of determining phase transition temperatures of phospholipids that constitute liposomes on the example of DPPC and DMPC. Thermochim. Acta 2014, 584, 36–44. [Google Scholar] [CrossRef]
- Davis, J. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys. J. 1979, 27, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Tenchov, B. On the reversibility of the phase transitions in lipid-Water systems. Chem. Phys. Lipids 1991, 57, 165–177. [Google Scholar] [CrossRef]
- Sun, L.; Böckmann, R.A. Membrane phase transition during heating and cooling: Molecular insight into reversible melting. Eur. Biophys. J. 2017, 47, 151–164. [Google Scholar] [CrossRef]
- Tsong, T.Y. Effect of phase transition on the kinetics of dye transport in phospholipid bilayer structures. Biochemistry 1975, 14, 5409–5414. [Google Scholar] [CrossRef]
- Ohyashiki, T.; Adachi, R.; Matsui, K. Changes in Surface Charge Density of Lecithin Liposomes by Lipid Peroxidation. A Fluorescence Study with 8-Anilino-1-Naphthalenesulfonate. Chem. Pharm. Bull. 1991, 39, 3295–3298. [Google Scholar] [CrossRef] [Green Version]
- Gasymov, O.K.; Glasgow, B.J. ANS fluorescence: Potential to augment the identification of the external binding sites of proteins. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2007, 1774, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Kosower, E.M.; Kanety, H. Intramolecular donor-Acceptor systems. 10. Multiple fluorescences from 8-(N-phenylamino)-1-Naphthalenesulfonates. J. Am. Chem. Soc. 1983, 105, 6236–6243. [Google Scholar] [CrossRef]
- Tsong, T.Y. Transport of 8-Anilino-1-Naphthalenesulfonate as a probe of the effect of cholesterol on the phospholipid bilayer structures. Biochemistry 1975, 14, 5415–5417. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Papahadjopoulos, D. Effect of a phase transition on the binding of 1-anilino-8-naphthalenesulfonate to phospholipid membranes. Biophys. J. 1976, 16, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Bhattachaya, S.; Haldar, S. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain-Backbone linkage. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1467, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Kraske, W.V.; Mountcastle, D.B. Effects of cholesterol and temperature on the permeability of dimyristoylphosphatidylcholine bilayers near the chain melting phase transition. Biochim. Biophys. Acta (BBA)—Biomembr. 2001, 1514, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Kindt, J.T. Simulation Study of the Permeability of a Model Lipid Membrane at the Fluid–Solid Phase Transition. Langmuir 2015, 31, 2187–2195. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Kamo, N.; Aiuchi, T.; Kurihara, K.; Kobatake, Y. The quantitative analysis of the interaction of 1-anilinonaphthalene-8-Sulfonate with liposomes: Fluorescence intensity and zeta-Potential. Colloid Polym. Sci. 1978, 256, 31–36. [Google Scholar] [CrossRef]
- Sierra, M.; Pedroni, V.; Buffo, F.; Disalvo, E.A.; Morini, M.A. The use of zeta potential as a tool to study phase transitions in binary phosphatidylcholines mixtures. Colloids Surf. B Biointerfaces 2016, 142, 199–206. [Google Scholar] [CrossRef]
- Morini, M.; Sierra, M.; Pedroni, V.; Alarcón, L.; Appignanesi, G.; Disalvo, E.A. Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles. Colloids Surf. B Biointerfaces 2015, 131, 54–58. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Siejak, P.; Pietralik, Z.; Kozak, M.; Polewski, K. Disruptive effect of tocopherol oxalate on DPPC liposome structure: DSC, SAXS, and fluorescence anisotropy studies. Chem. Phys. Lipids 2018, 216, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sturtevant, J.M. A scanning calorimetric study of small molecule-Lipid bilayer mixtures. Proc. Natl. Acad. Sci. USA 1982, 79, 3963–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, J.A.; Gawrisch, K. Effects of Ethanol on Lipid Bilayers Containing Cholesterol, Gangliosides, and Sphingomyelin. Biochemistry 1995, 34, 8852–8860. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.D.; Schawel, A.K.; Wang, W.; Dea, P. Effects of butanol-Isomers on dipalmitoylphosphatidylcholine bilayer membranes. Biophys. Chem. 2007, 128, 13–18. [Google Scholar] [CrossRef]
- Vierl, U.; Löbbecke, L.; Nagel, N.; Cevc, G. Solute effects on the colloidal and phase behavior of lipid bilayer membranes: Ethanol-Dipalmitoylphosphatidylcholine mixtures. Biophys. J. 1994, 67, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, S.; Wałejko, P. Synthesis of α-Tocopheryl Glycosides. Zeitschrift fur Naturforsch 2001, 56b, 411–415. [Google Scholar] [CrossRef]
- Witkowski, S.; Maciejewska, D.; Wawer, I. A solution and solid state conformations of chromanol esters, 13C MAS NMR and d-NMR study. J. Chem. Soc. Perkin Trans. 2000, 2, 1471–1476. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (TS, Toc) are available from the authors. |










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neunert, G.; Tomaszewska-Gras, J.; Witkowski, S.; Polewski, K. Tocopheryl Succinate-Induced Structural Changes in DPPC Liposomes: DSC and ANS Fluorescence Studies. Molecules 2020, 25, 2780. https://doi.org/10.3390/molecules25122780
Neunert G, Tomaszewska-Gras J, Witkowski S, Polewski K. Tocopheryl Succinate-Induced Structural Changes in DPPC Liposomes: DSC and ANS Fluorescence Studies. Molecules. 2020; 25(12):2780. https://doi.org/10.3390/molecules25122780
Chicago/Turabian StyleNeunert, Grażyna, Jolanta Tomaszewska-Gras, Stanislaw Witkowski, and Krzysztof Polewski. 2020. "Tocopheryl Succinate-Induced Structural Changes in DPPC Liposomes: DSC and ANS Fluorescence Studies" Molecules 25, no. 12: 2780. https://doi.org/10.3390/molecules25122780