Investigation of the Anticancer Activity of Coordination-Driven Self-AssembledTwo-Dimensional Ruthenium Metalla-Rectangle
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Preparation of Ruthenium Complex (1)
3.2. Synthesis of Metalla-Rectangle (3)
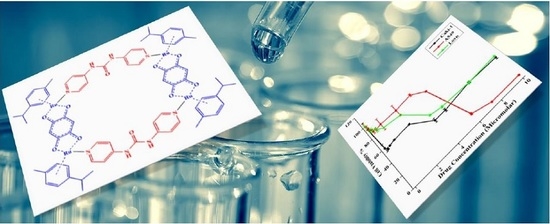
3.3. Stability of Metalla-Bowl in DMSO
3.4. UV-Vis Binding Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiteside, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. The discovery of crown ethers (Noble Lecture). Angew. Chem. Int. Ed. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry: Scope and Perspectives Molecules, supermolecules and molecular devices. Angew. Chem. Int. Ed. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Cram, D.J. The design of molecular hosts, guests, and their complexes (Noble Lecture). Angew. Chem. Int. Ed. 1988, 27, 1009–1020. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two-and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Oliveri, C.G.; Ulmann, P.A.; Wiester, M.J.; Mirkin, C.A. Heteroligated supramolecular coordination complexes formed via the halide-induced ligand rearrangement reaction. Acc. Chem. Res. 2008, 41, 1618–1629. [Google Scholar] [CrossRef]
- Harris, K.; Fujita, D.; Fujita, M. Giant hollow MnL2n spherical complexes: Structure, functionalization and applications. Chem. Comm. 2013, 49, 6703–6712. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Park, K.-M.; Lee, S.S. Metal, macrocycles and molecular assemblies-macrocyclic complexes in metallo-supramolecular chemistry. Chem. Soc. Rev. 2013, 42, 1713–1727. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.D. Polynuclear coordination cages. Chem. Commun. 2009, 30, 4487–4499. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Ni, Z.; O’Keeffe, M.; Yaghi, O.M. Reticular chemistry of metal-organic polyhedra. Angew. Chem. Int. Ed. 2008, 47, 5136–5147. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Gupta, R. Supramolecular architectures with pyridine-amide based ligands: Discrete molecular assemblies and their application. Dalton Trans. 2014, 43, 7668–7682. [Google Scholar] [CrossRef] [PubMed]
- Saalfrank, R.W.; Maid, H.; Scheurer, A. Supramolecular coordination chemistry: The synergistic effect of serendipity and rational design. Angew. Chem. Int. Ed. 2008, 47, 8794–8824. [Google Scholar] [CrossRef] [PubMed]
- Catti, L.; Zhang, Q.; Tiefenbacher, K. Advantages of Catalysis in Self-assembled molecular capsules. Chem.-Eur. J. 2016, 22, 9060–9066. [Google Scholar] [CrossRef] [PubMed]
- Koblenz, T.S.; Wassenaar, J.; Reek, J.N.H. Reactivity within a confined self-assembled nanospace. Chem. Soc. Rev. 2008, 37, 247–262. [Google Scholar] [CrossRef]
- Meeuwissen, J.; Reek, J.N.H. Supramolecular catalysis beyond enzyme mimics. Nat. Chem. 2010, 2, 615–621. [Google Scholar] [CrossRef]
- Howlader, P.; Das, P.; Zangrando, E.; Mukherjee, P.S. Urea functionalized self-assembled molecular prism for heterogeneous catalysis in water. J. Am. Chem. Soc. 2016, 138, 1668–1676. [Google Scholar] [CrossRef]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-assembled metal-organic polyhedra: An overview of various applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Vardhan, H.; Verpoort, F. Metal-organic polyhedra: Catalysis and reactive intermediates. Adv. Synth. Catal. 2015, 357, 1351–1368. [Google Scholar] [CrossRef]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Proton-mediated chemistry and catalysis in a self-assembled supramolecular host. Acc. Chem. Res. 2009, 42, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Lippard, S.J. Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery. Chem. Sci. 2015, 6, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-R.; Suntharalingam, K.; Bruno, P.M.; Lin, W.; Wang, W.; Hemann, M.T.; Lippard, S.J. Mechanistic studies of the anticancer activity of an octahedral hexanuclear Pt(II) cage. Inorg. Chim. Acta 2016, 452, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Therrein, B. Transporting and shielding photosensitizers by using water-soluble organometallic cages: A new strategy in drug delivery and photodynamic therapy. Chem.-Eur. J. 2013, 19, 8378–8386. [Google Scholar] [CrossRef] [PubMed]
- Therrein, B. Biologically relevant arene ruthenium metalla-assemblies. CrystEngComm 2015, 17, 484–491. [Google Scholar] [CrossRef]
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.-W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef]
- Therrien, B. Drug delivery by water-soluble organometallic cages. Top. Curr. Chem. 2011, 319, 35–55. [Google Scholar]
- Barry, N.P.E.; Zava, O.; Dyson, P.J.; Therrien, B. Synthesis, Characterization and Anticancer activity of porphyrin-containing organometallic cubes. Aust. J. Chem. 2010, 63, 1529–1537. [Google Scholar] [CrossRef]
- Therrien, B. Arene ruthenium cages: Boxes full of surprise. Eur. J. Inorg. Chem. 2009, 2009, 2445–2453. [Google Scholar] [CrossRef]
- Nakamura, T.; Ube, H.; Shionoya, M. Silver-mediated formation of a cofacial porphyrin dimer with the ability to intercalate aromatic molecules. Angew. Chem. Int. Ed. 2013, 52, 12096–12100. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.A.L.; Gong, J.Q.; Haver, R.; Odell, B.; Claridge, T.D.W.; Herz, L.M.; Anderson, H.L. Self-assembly of Russian doll concentric porphyrin nanorings. J. Am. Chem. Soc. 2015, 137, 12713–12718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Z.; Han, Y.-F.; Lin, Y.-J.; Jin, G.-X. Synthesis and characterization of molecular rectangles of half-sandwich p-cymene ruthenium complexes bearing oxamidato ligands. Dalton Trans. 2009, 39, 8426–8431. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-F.; Fei, Y.; Jin, G.-X. Self-assembled half-sandwich Ir, Rh-based organometallic molecular boxes for reversible trapping of halocarbon molecules. Dalton Trans. 2010, 39, 3976–3984. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, V.; Song, Y.H.; Lee, M.H.; Kim, H.; Wang, M.; Stang, P.J.; Chi, K.-W. Self-assembled arene-ruthenium-based rectangles for the selective sensing of multi-carboxylate anions. Chem. Eur. J. 2011, 17, 7837–7844. [Google Scholar] [CrossRef] [PubMed]
- Furrer, M.A.; Furrer, J.; Therrien, B. Physical and physicochemical stimuli-responsive arene ruthenium metallaprisms. Organometallics 2012, 31, 3149–3154. [Google Scholar] [CrossRef]
- Mishra, A.; Vajpayee, V.; Kim, H.; Lee, M.H.; Jung, H.; Wang, M.; Stang, P.; Chi, K.-W. Self-assembled metalla-bowls for selective sensing of multi-carboxylate anions. Dalton Trans. 2012, 41, 1195–1201. [Google Scholar] [CrossRef]
- Wang, M.; Vajpayee, V.; Shanmugaraju, S.; Zheng, Y.R.; Zhao, Z.; Kim, H.; Mukherjee, P.S.; Chi, K.-W.; Stang, P.J. Coordination-driven self-assembly of M3L2 trigonal cages from preorganized metalloligands incorporating octahedral metal centers and fluorescent detection of nitroaromatics. Inorg. Chem. 2011, 50, 1506–1512. [Google Scholar] [CrossRef]
- Tehrani, A.A.; Esrafili, L.; Abedi, S.; Morsalim, A.; Carlucci, L.; Proserpio, D.M.; Wang, J.; Junk, O.C.; Liu, T. Urea Metal-organic frameworks for nitro-substituted compounds sensing. Inorg. Chem. 2017, 56, 1446–1454. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Furrer, J.; Freudenreich, J.; Süss-Fink, G.; Therrien, B. Designing the host-guest properties of tetranuclear arene ruthenium metalla-rectangles to accommodate pyrene molecule. Eur. J. Inorg. Chem. 2010, 2010, 725–728. [Google Scholar] [CrossRef]
- Govender, P.; Renfrew, A.K.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. Antiproliferative activity of chelating N,O- and N,N-ruthenium (II) arene functionalized poly(propyleneimine) dendrimer scaffolds. Dalton Trans. 2011, 40, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, V.; Lee, S.; Kim, S.-H.; Kang, S.C.; Cook, T.R.; Kim, H.; Kim, D.W.; Verma, S.; Lah, M.S.; Kim, I.S.; et al. Self-assembled metalla-rectangles bearing azodipyridyl ligands: Synthesis, characterization and antitumor activity. Dalton Trans. 2013, 42, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Oldacre, A.N.; Friedman, A.E.; Cook, T.R. A self-assembled cofacial cobalt porphyrin prism for oxygen reduction catalysis. J. Am. Chem. Soc. 2017, 139, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Mehta, A.; Nath, I.; Verpoort, F. Dynamic imine chemistry in metal-organic polyhedra. RSC Adv. 2015, 5, 67011–67030. [Google Scholar] [CrossRef]
- Caltagirone, C.; Gale, P.A. Anion receptor chemistry: Highlights from 2007. Chem. Soc. Rev. 2009, 38, 520–563. [Google Scholar] [CrossRef]
- Gale, P.A.; Quesada, R. Anion coordination and anion-templated assembly: Highlights from 2002 to 2004. Coord. Chem. Rev. 2006, 250, 3219–3244. [Google Scholar] [CrossRef]
- Oshovsky, G.V.; Reinhoudt, D.N.; Verboom, W. Supramolecular chemistry in water. Angew. Chem. Int. Ed. 2007, 46, 2366–2393. [Google Scholar] [CrossRef]
- Gales, P.A.; Garcia-Garrido, S.E.; Garric, J. Anion receptor based on organic frameworks: Highlights from 2005 and 2006. Chem. Soc. Rev. 2008, 37, 151–190. [Google Scholar] [CrossRef]
- Morakot, N.; Rakrai, W.; Keawwangchai, S.; Kaewtong, C.; Wanno, B. Design and synthesis of thiourea based receptor containing naphthalene as oxalate selective sensor. J. Mol. Model. 2009, 16, 129–136. [Google Scholar] [CrossRef]
- Pal, R.; Parker, D.; Costello, L.C. A europium luminescence assay of lactate and citrate in biological fluid. Org. Biomol. Chem. 2009, 7, 1525–1528. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Prostatic fluid electrolyte composition for the screening of prostate cancer: A potential solution to a major problem. Prostate Cancer Prostatic Dis. 2008, 12, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, V.; Yang, Y.J.; Kang, S.C.; Kim, H.; Kim, I.S.; Wang, M.; Stang, P.J.; Chi, K.-W. Hexanuclear self-assembled arene-ruthenium nanoprismatic cages: Potential anticancer agents. Chem. Commun. 2011, 47, 5184–5186. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Edafe, F.; Therrien, B. Anticancer activity of tetracationic arene ruthenium metalla-cycles. Dalton Trans. 2011, 40, 7172–7180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, N.P.E.; Zava, P.; Furrer, J.; Dyson, P.J.; Therrien, B. Anticancer activity of opened arene ruthenium metalla-assemblies. Dalton Trans. 2010, 39, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Therrien, B.; Süss-Fink, G.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J. The “complex-in-a-complex” cations [(acac)2M⊂Ru6(p-iPrC6H4Me)6(tpt)2(dhbq)3]6+: A Trojan Horse for cancer cells. Angew. Chem. Int. Ed. 2008, 47, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Zava, O.; Mattsson, J.; Therrien, B.; Dyson, P.J. Evidence for drug release from a metalla-cage delivery vector following cellular internalization. Chem. Eur. J. 2010, 16, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Abd Karim, N.H.; Vilar, R.; Therrien, B. Interactions of ruthenium coordination cubes with DNA. Dalton Trans. 2009, 10717–10719. [Google Scholar] [CrossRef]
- Vajpayee, V.; Song, Y.H.; Yang, Y.J.; Kang, S.C.; Cook, T.R.; Kim, D.W.; Lah, M.S.; Kim, I.S.; Wang, M.; Stang, P.J.; et al. Self-assembly of cationic, hetero-or homonuclear ruthenium (II) macrocyclic rectangles and their photophysical, electrochemical, and biological studies. Organometallics 2011, 30, 6482–6489. [Google Scholar] [CrossRef]
- Mishra, A.; Jung, H.; Park, J.W.; Kim, H.K.; Kim, H.; Stang, P.J.; Chi, K.-W. Anticancer activity of Self-assembled molecular rectangles via arene-ruthenium acceptors and a new unsymmetrical amide ligand. Organometallics 2012, 31, 3519–3526. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host-guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Linares, F.; Galindo, M.A.; Galli, S.; Romero, M.A.; Navarro, J.A.R.; Barea, E. Tetranuclear coordination assemblies based on half-sandwich ruthenium (II) complexes: Noncovalent binding to DNA and cytotoxicity. Inorg. Chem. 2009, 48, 7413–7420. [Google Scholar] [CrossRef] [PubMed]
- Linares, F.; Procopio, E.Q.; Galindo, M.A.; Romero, M.A.; Navarro, J.A.R.; Barea, E. Molecular architecture of redox-active half-sandwich Ru(II) cyclic assemblies. Interaction with biomolecules and anticancer activity. CrystEngComm 2010, 12, 2343–2346. [Google Scholar] [CrossRef]
- Dubey, A.; Jeong, Y.J.; Jo, J.H.; Woo, S.; Kim, D.H.; Kim, H.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Anticancer activity and autophagy involvement of self-assembled arene-ruthenium metallacycles. Organometallics 2015, 34, 4507–4514. [Google Scholar] [CrossRef]
- Keller, W.E. Evidence for the planar structure of the urea molecule. J. Chem. Phys. 1948, 16, 1003. [Google Scholar] [CrossRef]
- Platts, J.A.; Maarof, H.; Harris, K.D.M.; Lim, G.K.; Willock, D.J. The effect of intermolecular hydrogen bonding on the planarity of amides. Phys. Chem. Chem. Phys. 2012, 14, 11944–11952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Süss-Fink, G.; Neels, A.; Stoeckli-Evans, H. Mono-, di- and tetra-nuclear p-cymene ruthenium complexes containing oxalate ligands. J. Chem. Soc. Dalton Trans. 1997, 22, 4345–4350. [Google Scholar]
- Vardhan, H.; Verpoort, F. Ligand constraints and synthesis of metal-organic polyhedra. Aust. J. Chem. 2015, 68, 707–730. [Google Scholar] [CrossRef]
- Paul, L.E.H.; Therrien, B.; Furrer, J. Interactions of arene ruthenium metallaprisms with human proteins. Org. Biomol. Chem. 2015, 13, 946–953. [Google Scholar] [CrossRef]
- Vajpayee, V.; Lee, S.M.; Park, J.W.; Dubey, A.; Kim, H.; Cook, T.R.; Stang, P.J.; Chi, K.-W. Growth inhibitory activity of a bis-benzimidazole-bridged arene ruthenium metalla-rectangle and -prims. Organometallics 2013, 32, 1563–1566. [Google Scholar] [CrossRef]
- Orhan, E.; Garci, A.; Therrien, B. Coordination-driven self-assembly of arene ruthenium metalla-rectangles. Inorg. Chim. Acta 2017, 461, 78–83. [Google Scholar] [CrossRef]
- Orhan, R.; Garci, A.; Therrien, B. Flexible arene ruthenium metalla-prisms. Inorg. Chim. Acta 2015, 438, 5–9. [Google Scholar] [CrossRef]
- Johnson, C.S., Jr. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E.; Zava, O.; Deschenaux, R.; Dyson, P.J.; Therrien, B. Double targeting of tumours with pyrenyl-modified dendrimers encapsulated in an arene-ruthenium metalla-prism. Chem. Eur. J. 2011, 17, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Elumalai, P.; Singh, N.; Kim, H.; Lee, S.U.; Chi, K.-W. Selective synthesis of ruthenium (II)metalla[2]catenane via solvent and guest-dependent self-assembly. J. Am. Chem. Soc. 2015, 137, 4674–4677. [Google Scholar] [CrossRef] [PubMed]
- Percástegui, E.G.; Mosquera, J.; Nitschke, J.R. Anion exchange renders hydrophobic capsule and cargoes water-soluble. Angew. Chem. Int. Ed. 2017, 56, 9136–9140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zangrando, E.; Mukherjee, P.S. Self-assembled Pd3L2 cages having flexible tri-imidazole donors. Polyhedron 2019. [Google Scholar] [CrossRef]
- Okan, S.E.; Champeney, D.C. Molar conductance of aqueous solutions of sodium, potassium, and nickel trifluoromethanesulfonate at 25 °C. J. Solut. Chem. 1997, 26, 405–414. [Google Scholar] [CrossRef]
- Mishra, A.; Jeong, Y.J.; Jo, J.-H.; Kang, S.C.; Lah, M.S.; Chi, K.-W. Anticancer potency studies of coordination driven self-assembled arene-Ru-based metalla-bowls. ChemBioChem 2014, 15, 695–700. [Google Scholar] [CrossRef]
- Mishra, A.; Lee, S.C.; Kaushik, N.; Cook, T.R.; Choi, E.H.; Kaushik, N.K.; Stang, P.J.; Chi, K.-W. Self-assembled supramolecular hetero-bimetallacycles for anticancer potency by intracellular release. Chem. Eur. J. 2014, 20, 14410–14420. [Google Scholar] [CrossRef]
- Vardhan, H.; Mehta, A.; Ezugwu, C.I.; Verpoort, F. Self-assembled arene ruthenium metalla-assemblies. Polyhedron 2016, 112, 104–108. [Google Scholar] [CrossRef]
- Vardhan, H.; Verpoort, F. UV-Vis absorption studies of coordination-driven self-assembled 2D metalla-rectangle towards multi-carboxylation anions. Polyhedron 2019, 157, 262–266. [Google Scholar] [CrossRef]
- Vajpayee, V.; Song, Y.H.; Jung, Y.J.; Kang, S.C.; Kim, H.; Kim, I.S.; Wang, M.; Cook, T.R.; Stang, P.J.; Chi, K.-W. Coordination-driven self-assembly of ruthenium-based molecular-rectangles: Synthesis, characterization, photophysical and anticancer potency studies. Dalton Trans. 2012, 41, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Therrein, B. The role of the second coordination sphere in the biological activity of arene ruthenium metalla-assemblies. Front. Chem. 2018, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Hengartner, M.O. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef]
- Jing, H.; Dubey, A.; Koo, H.J.; Vajpayee, V.; Cook, T.R.; Kim, H.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Self-assembly of ambidentate pyridyl-carboxylate ligands with octahedral ruthenium metal centers: Self-selection for a single-linkage isomer and anticancer-potency studies. Chem. Eur. J. 2013, 19, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Min, J.W.; Koo, H.J.; Kim, H.; Cook, T.R.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Anticancer potency and multidrug-resistant studies of self-assembled arene-ruthenium metallarectangles. Chem. Eur. J. 2013, 19, 11622–11628. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1,2 and 3 are available from the authors. |





| Cell Lines | IC50, µMA | Cisplatin | Ruthenium Complex (1) |
|---|---|---|---|
| LOVO | 3.2 ± 1.13 | 7.1 ± 0.67 | >100 |
| CAKI-1 | 3.3 ± 1.21 | >100 | |
| A549 | 4.0 ± 1.28 | >100 | >100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardhan, H.; Nafady, A.; Al-Enizi, A.M.; Khandker, K.; El-Sagher, H.M.; Verma, G.; Acevedo-Duncan, M.; Alotaibi, T.M.; Ma, S. Investigation of the Anticancer Activity of Coordination-Driven Self-AssembledTwo-Dimensional Ruthenium Metalla-Rectangle. Molecules 2019, 24, 2284. https://doi.org/10.3390/molecules24122284
Vardhan H, Nafady A, Al-Enizi AM, Khandker K, El-Sagher HM, Verma G, Acevedo-Duncan M, Alotaibi TM, Ma S. Investigation of the Anticancer Activity of Coordination-Driven Self-AssembledTwo-Dimensional Ruthenium Metalla-Rectangle. Molecules. 2019; 24(12):2284. https://doi.org/10.3390/molecules24122284
Chicago/Turabian StyleVardhan, Harsh, Ayman Nafady, Abdullah M. Al-Enizi, Khalid Khandker, Hussein M. El-Sagher, Gaurav Verma, Mildred Acevedo-Duncan, Tawfiq M. Alotaibi, and Shengqian Ma. 2019. "Investigation of the Anticancer Activity of Coordination-Driven Self-AssembledTwo-Dimensional Ruthenium Metalla-Rectangle" Molecules 24, no. 12: 2284. https://doi.org/10.3390/molecules24122284