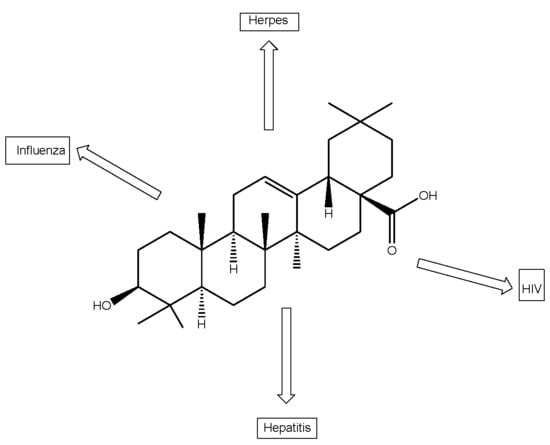
Antiviral Activities of Oleanolic Acid and Its Analogues
Abstract
:1. Introduction
Analogues of Oleanolic Acid
2. Phytochemical Studies and Anti-Viral Activities of OA
2.1. Anti-HIV Activity
2.2. Anti-Influenza
2.3. Anti-Hepatitis
2.4. Anti-Herpes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rumlová, M.; Ruml, T. In vitro methods for testing antiviral drugs. Biotechnol. Adv. 2018, 36, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Nováková, L.; Pavlík, J.; Chrenková, L.; Martinec, O.; Červený, L. Current antiviral drugs and their analysis in biological materials—Part I: Antivirals against respiratory and herpes viruses. J. Pharm. Biomed. Anal. 2018, 147, 400–416. [Google Scholar] [CrossRef] [PubMed]
- National Health Laboratory Service. Available online: http://www.nhls.ac.za/?page=alerts&id=5&archive=2016&rows=5&pager=5 (accessed on 5 November 2017).
- Kitazato, K.; Wang, Y.; Kobayashi, N. Viral infectious disease and natural products with antiviral activity. Drug Discov. Ther. 2007, 1, 14–22. [Google Scholar] [PubMed]
- Ganjhu, R.K.; Mudgal, P.P.; Maity, H.; Dowarha, D.; Devadiga, S.; Nag, S.; Arunkumar, G. Herbal plants and plant preparations as remedial approach for viral diseases. VirusDisease 2015, 26, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed-Belkacem, A.; Ahnou, N.; Barbotte, L.; Wychowski, C.; Pallier, C.; Brillet, R.; Pohl, R.; Pawlotsky, J. Silibinin and Related Compounds Are Direct Inhibitors of Hepatitis C. Gastroenterology 2010, 138, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Morishima, C.; Shuhart, M.C.; Wang, C.C.; Paschal, D.M.; Apodaca, M.C. Silymarin Inhibits In Vitro T-Cell Proliferation and Cytokine Production in Hepatitis C Virus Infection. Gastroenterology 2010, 138, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Moons, N.; Kim, Y.; Borggraeve, W.D.; Mashentseva, A.; Andrei, G.; Snoeck, R.; Balzarini, J.; Dehaen, W. Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities. Bioorg. Med. Chem. 2014, 22, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L. The isoprene rule and the biogenesis of terpenic compounds. Experientia 1953, 9, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.G.; Feijoo-Siota, L.; Rama, J.L.R.; Ageitos, J.M. Antivirals against animal viruses. Biochem. Pharmacol. 2017, 133, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Pompei, R.; Flore, O.; Marccialis, A.M.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Qian, S.; Yoshiki, K.; Zhang, D.-C.; Hu, C.-Q.; Jin, J.-Q.; Nozaki, H.; Kilkuskie, R.E.; Tramontano, E.; Cheng, Y.-C.; et al. Anti-aids agents, 6. salaspermic acid, an anti-HIV principle from tripterygium wilfordii, and the structure-activity correlation with its related compounds. Prod. J. Nat. 1992, 55, 340–346. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Robert, E.K.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Lanzen, W.P.; Cheen, I.; Lee, K. Anti- aids agents, 11 betulinic acid and plantanic acid as anti-HIV principles from syzygium claviflorum, and the anti-HIV activity of structurally relate triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; Zhu, Y. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antiviral Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Rali, S.; Oyedeji, O.O.; Aremu, O.O.; Oyedeji, A.O.; Nkeh-Chungag, B.N. Semisynthesis of derivatives of oleanolic acid from Syzygium aromaticum and their antinociceptive and anti-inflammatory properties. Mediators Inflamm. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Lee, S.; Yoon, Y.; Choi, K.H. Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS ONE 2015, 10, e0118800. [Google Scholar] [CrossRef] [PubMed]
- Hichri, F.; Ben, H.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chim. 2003, 6, 473–483. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Zhang, P.; Zhu, H.; Chen, L.; Xiao, Y.; Xing, Y. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumor Biol. 2016, 37, 7599–7613. [Google Scholar] [CrossRef] [PubMed]
- Chouaïb, K.; Romdhane, A.; Dlelmasure, S.; Dutartre, P.; Elie, N.; Toutboul, D.; Ben, H.; Ali, M. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3,5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299. [Google Scholar] [CrossRef]
- Guo, Y.; Han, B.; Luo, K.; Ren, Z.; Cai, L.; Sun, L. NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation. Biomed. Pharmacother. 2017, 85, 733–773. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B. K.H.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Critea, M.; Ivan, A.; Dancui, C.; Dehelean, C.; et al. Improvement of ursolic and oleanolic acids′ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed. Pharmacother. 2015, 83, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, B.; Nayak, L.V.; Sistla, R.; Mallavadhani, V.U. Bioorganic Chemistry Synthesis of ring-C modified oleanolic acid derivatives and their cytotoxic evaluation. Bioorg. Chem. 2016, 68, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, L.; Cheng, N.; Jia, M.; Zhang, Y. Extraction optimization of oleanolic and ursolic acids from pomegranate (Punica granatum L.) flowers. Food Bioprod. Process. 2014, 92, 321–327. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crops Prod. 2016, 84, 72–79. [Google Scholar] [CrossRef]
- Chouab, K.; Hichri, F.; Nguir, A.; Daami-Remadi, M.; Elie, N.; Touboul, D.; Ben, J.H.; Hamza, M. Semi-synthesis of new antimicrobial esters from the natural oleanolic and maslinic acids. Food Chem. 2015, 183, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Weckesser, S.; Engel, K.; Simon-haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2007, 14, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.A.; Abu-salah, K.M.; Salman, A. Syzygium aromaticum extracts as good source of betulinic acid and potential anti-breast cancer. Rev. Bras. Farmacogn. 2012, 22, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zeng, H.; Wang, Y.; Fan, X.; Xu, C.; Deng, R.; Zhou, X.; Bi, H.; Huang, M. Low Dose of Oleanolic Acid Protects against Lithocholic Acid–Induced Cholestasis in Mice: Potential Involvement of Nuclear Factor-E2-Related Factor 2-Mediated Upregulation of Multidrug Resistance-Associated Proteins. Am. Soc. Pharmacol. Exp. Ther. 2014, 42, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A Subfamily Members are Multifunctional Oxidases in Triterpenoid Biosynthesis. Plant Cell Physiol. 2011, 52, 2050–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Strehle, A.; Thomas, C.; Sato, H.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar]
- Sánchez, M.; Theoduloz, C.; Schmeda-hirschmann, G.; Razmilic, I.; Yáñez, T.; Rodríguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In vitro–in vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Banik, R.M.; Pandey, D.K. Optimizing conditions for oleanolic acid extraction from Lantana camara roots using response surface methodology. Ind. Crops Prod. 2008, 27, 241–248. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, K. Solubility Studies of Oleanolic Acid and Betulinic Acid in Aqueous Solutions and Plant Extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.J.; Soumyanath, A. Amylase inhibitory activity of some Malaysian plants used to treat diabetes with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Li, Y.; He, J.; Cheng, X.; Wang, K.; Li, M.M.; Pan, Z.H.; Peng, L.Y.; Zhao, Q.S. Triterpenoids and diterpenoids from Viburnum chingii. Chem. Pharm. Bull. 2011, 59, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Niampoka, C.; Suttisri, R.; Bavovada, R.; Takayama, H.; Aimi, N. Potentially cytotoxic triterpenoids from the root bark of Siphonodon celastrineus Griff. Arch. Pharm. Res. 2005, 28, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Kaweetripob, W.; Mahidol, C.; Prawat, H.; Ruchirawat, S. Lupane, friedelane, oleanane, and ursane triterpenes from the stem of Siphonodon celastrineus Griff. Phytochemistry 2013, 96, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhu, Y.; Zhang, H.J.; Jiao, W.H.; Han, B.N.; Liu, Z.X.; Qiu, F.; Chen, W.S.; Lin, H.W. Anti-inflammatory secondary metabolites from the leaves of Rosa laevigata. Bioorg. Med. Chem. 2013, 21, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Sporn, M.B. Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, N.; Wng, Y.; Honda, T.; Gribble, G.W. Advances in Brief A Novel Synthetic Oleanane Triterpenoid, 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic Acid, with Potent Differentiating, Antiproliferative, and Anti-Inflammatory Activity. Cancer Res. 1999, 59, 336–341. [Google Scholar] [PubMed]
- Chen, J.; Liu, J.; Zhang, L.; Wu, G.; Hua, W. Pentacyclic triterpenes. Part 3: Synthesis and biological evaluation of oleanolic acid derivatives as novel inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. Lett. 2006, 16, 2915–2919. [Google Scholar] [CrossRef] [PubMed]
- Pollier, J.; Goossens, A. Phytochemistry Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nkeh-chungag, B.N.; Oyedeji, O.O.; Oyedeji, A.O.; Ndebia, E.J. Anti-Inflammatory and Membrane-Stabilizing Properties of Two Semisynthetic Derivatives of Oleanolic Acid. Inflammation 2015, 38, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Holmes, S.S.; Baker, G.A.; Challa, S.; Bose, H.S.; Song, Z. Ionic derivatives of betulinic acid as novel HIV-1 protease inhibitors. J. Enzyme Inhib. Med. Chem. 2012, 27, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.; Chen, C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005, 11, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Sriram, D. Betulinic Acid and Its Derivatives: A Review on their Biological Properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, J.; Wang, H.; Mark, L.; Lee, K. Synthesis and Anti-HIV Activity of Oleanolic Acid Derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 3115–3118. [Google Scholar] [CrossRef]
- Yu, D.; Sakurai, Y.; Chen, C.; Chang, F.; Huang, L.; Kashiwad, Y.; Kuo-Hsiung, L. Anti-AIDS Agents 69. Moronic Acid and Other Triterpene Derivatives as Novel Potent Anti-HIV Agents. J. Med. Chem. 2006, 49, 5462–5469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwada, Y.; Nagao, T.; Hashimoto, A.; Ikeshiro, Y.; Okabe, H.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J. Nat. Prod. 2000, 63, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Wang, H.K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H.; et al. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzyme Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Song, D.; Poo, H. Antiviral Activity of the Plant Extracts from Thuja orientalis, Aster spathulifolius, and Pinus thunbergii against Influenza Virus A/PR/8/34. J. Microbiol. Biotechnol. 2013, 23, 125–130. [Google Scholar]
- Lee, J.; Miyake, S.; Umetsu, R.; Hayashi, K.; Chijimatsu, T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.). Food Chem. 2012, 134, 2164–2168. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, E.; Hayashi, K.; Katayama, H.; Hayashi, T.; Obata, A. Anti-Influenza Virus Effects of Elderberry Juice and Its Fractions. Biosci. Biotechnol. Biochem. 2012, 76, 1633–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriwilaijaroen, N.; Fukumoto, S.; Kumagai, K.; Hiramatsu, H.; Odagiri, T. Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: Its role in viral hemagglutination and neuraminidase inhibition. Antiviral Res. 2012, 94, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, W.; Zhao, A.; Wang, X. Anti-influenza agents from plants and traditional Chinese medicine. Phyther. Res. 2006, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, J.; Li, W.; Wang, X.; Xu, J.; Xie, J.; Tao, K.; Shen, L.; Zhang, R. Molecular docking of potential inhibitors for influenza H7N9. Comput. Math. Methods Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, H.; Chang, H.; Yu, Y.; Yang, M.; He, Y. Multivalent oleanolic acid human serum albumin conjugate as nonglycosylated neomucin for influenza virus capture and entry inhibition. Eur. J. Med. Chem. 2018, 143, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shi, Y.; Si, L.; Fan, Z.; Wang, H.; Xu, R.; Jiao, P.; Meng, K.; Tian, Z.; Zhou, X.; et al. Design, synthesis and biological activity evaluation of novel conjugated sialic acid and pentacyclic triterpene derivatives as anti-influenza entry inhibitors. MedChemComm 2016, 7, 1932–1945. [Google Scholar] [CrossRef]
- Han, X.; Si, L.L.; Shi, Y.Y.; Fan, Z.B.; Wang, S.X.; Tian, Z.Y.; Li, M.; Sun, J.Q.; Jiao, P.X.; Ran, F.X.; et al. Synthesis and in vitro anti-influenza virus evaluation of novel sialic acid (C-5 and C-9)-pentacyclic triterpene derivatives. Molecules 2017, 22, 1018. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Ma, C.; Wei, Y.; Salah, R.; Dine, E.; Sato, N. Survey of Anti-HIV and Anti-HCV Compounds from Natural Sources. Can. Chem. Trans. 2013, 1, 116–140. [Google Scholar]
- Pastuch-Gawolek, G.; Chaubey, B.; Szewczyk, B.; Krol, E. Novel thioglycosyl analogs of glycosyltransferase substrates as antiviral compounds against classical swine fever virus and hepatitis C virus. Eur. J. Med. Chem. 2017, 137, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, C.; Li, B.; Xu, X.; Liang, M.; Gu, S.; Chu, S.; Xu, B.; Ren, J.; Wang, P.; et al. A Series of Oleanolic Acid Derivatives as Anti-Hepatitis B Virus Agents: Design, Synthesis, and in Vitro and in Vivo Biological Evaluation. Molecules 2016, 21, 402. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, H.; Ojha, D.; Bag, P.; Chandel, H.S.; Bhattacharyya, S.; Chatterjee, T.K.; Mukherjee, P.K.; Chakraborti, S.; Chattopadhyay, D. Anti-herpes virus activities of Achyranthes aspera: An Indian ethnomedicine, and its triterpene acid. Microbiol. Res. 2013, 168, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004, 30, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Keda, T.I.; Okomizo, K.Y.; Suchihashi, M.O.; Injo, J.K. Anti-herpes Virus Type 1 Activity of Oleanane-Type Triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [Green Version]
- Sarrazin, C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J. Hepatol. 2016, 64, 486–504. [Google Scholar] [CrossRef] [PubMed]












| Plant Species (Family) | Biological Activity | Plant Parts Used | References |
|---|---|---|---|
| Oleaeuropaea L. (Oleaceae) | Anticancer, antimicrobial, anti-diabetic | Fruits and leaves | [22,28,37,38] |
| Fabiana imbricata R. et P. (Solanaceae) | Antiviral, antitumor, and antihyperlipidemic | Leaves and flowers | [39] |
| Syzygium aromaticum (Myrtaceae) | Antinociceptive, Anti-inflammatory, antihypertensive, and antioxidant | Flower buds and leaves | [18,39,40] |
| Ligustrum lucidum Ait (Oleaceae) | Anti-inflammatory, antioxidative, antiprotozoal, antimutagenic, and anticancer | Fruits and leaves | [41] |
| Viscum album (Santalaceae) | Anti-tumor, analgesic, and anti-inflammatory | Leaves and stems | [19,41,42] |
| Phyllanthus amarus (Phyllanthaceae) | Anti-diabetes | Leaves or aerial | [43] |
| Punica granatum L. (Punicaceae) | Antioxidant activity | Fruit | [27] |
| Rosmarinus officinalis L. (Lamiaceae) | Anti-inflammatory, hepatoprotective, gastroprotective, antiulcer | Leaves, flowers, stems, branches. | [28] |
| Gentiana lutea (Gentianaceae) | Antimicrobial | Dried root and rhizome | [30] |
| L. camara (Verbenaceae) | Anti-inflammatory, antioxidative, antiprotozoal | Leaves and flowers | [41] |
| Viburnum chingii (Asteraceae) | Antimicrobial | Leaves | [44] |
| Siphonodon celastrineus (Celastraceae) | Anti-inflammatory | Root bark, stem | [45,46] |
| Rosa laevigata (Rosaceae) | Anti-inflammatory | Leaves | [47] |
| Fructus Ligustri Lucidi (FLL) | Anti-hepatitis | Leaves | [18] |
| Compound | R | X | Yield (%) 1 |
|---|---|---|---|
| 15 |  | RCl | 98 |
| 16 |  | RCl | 94 |
| 17 |  | RCl | 91 |
| 18 |  | RCl | 95 |
| 19 |  | RCl | 94 |
| 20 |  |  | 92 |
| 21 |  |  | 82 |
| 22 |  |  | 91 |
| 23 |  |  | 85 |
| 24 |  |  | 81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. https://doi.org/10.3390/molecules23092300
Khwaza V, Oyedeji OO, Aderibigbe BA. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules. 2018; 23(9):2300. https://doi.org/10.3390/molecules23092300
Chicago/Turabian StyleKhwaza, Vuyolwethu, Opeoluwa O. Oyedeji, and Blessing A. Aderibigbe. 2018. "Antiviral Activities of Oleanolic Acid and Its Analogues" Molecules 23, no. 9: 2300. https://doi.org/10.3390/molecules23092300