Antimicrobial Lemongrass Essential Oil—Copper Ferrite Cellulose Acetate Nanocapsules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dynamic Light Scattering
2.2. X-ray Diffraction
2.3. Atomic Force Microscopy
2.4. High Resolution Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy and Transmission Electron Microscopy
2.5. Magnetic Measurements
2.6. Raman Spectroscopy
2.7. UV-VIS Absorption Spectroscopy
2.8. Inductively Coupled Plasma Mass Spectrometry
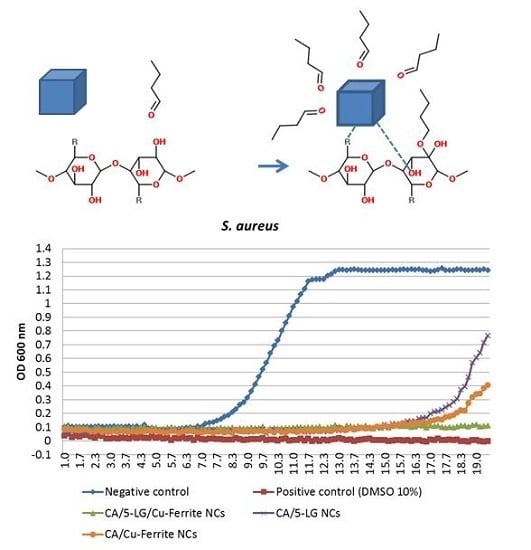
2.9. Antimicrobial Tests
3. Experimental Section
3.1. Materials
3.2. Cellulose Acetate/Lemongrass Oil/Cu-Ferrite Nanocapsules Preparation
3.3. Dynamic Light Scattering
3.4. X-ray Diffraction
3.5. Atomic Force Microscopy
3.6. High Resolution Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy and Transmission Electron Microscopy
3.7. Magnetic Measurements
3.8. Raman Spectroscopy
3.9. UV-VIS Absorption Spectroscopy
3.10. Inductively Coupled Plasma Mass Spectrometry
3.11. Antimicrobial Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and applications of cellulose acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Q.; Ren, X.; Xie, Z.; Huang, T.-S. Electrospun non-leaching biocombatible antimicrobial cellulose acetate nanofibrous mats. J. Ind. Eng. Chem. 2015, 27, 315–321. [Google Scholar] [CrossRef]
- Ohkawa, K. Nanofibers of cellulose and its derivatives fabricated using direct electrospinning. Molecules 2015, 20, 9139. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Kargl, R.; Tradt, K.E.; Kulterer, M.R.; Braćić, M.; Hribernik, S.; Stana-Kleinschek, K.; Ribitsch, V. Antifouling coating of cellulose acetate thin films with polysaccharide multilayers. Carbohydr. Polym. 2015, 116, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kulterer, M.R.; Reischl, M.; Reichel, V.E.; Hribernik, S.; Wu, M.; Köstler, S.; Kargl, R.; Ribitsch, V. Nanoprecipitation of cellulose acetate using solvent/nonsolvent mixtures as dispersive media. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 23–29. [Google Scholar] [CrossRef]
- Kulterer, M.R.; Reichel, V.E.; Kargl, R.; Köstler, S.; Sarbova, V.; Heinze, T.; Stana-Kleinschek, K.; Ribitsch, V. Functional polysaccharide composite nanoparticles from cellulose acetate and potential applications. Adv. Funct. Mater. 2012, 22, 1749–1758. [Google Scholar] [CrossRef]
- Liakos, I.L.; D’autilia, F.; Garzoni, A.; Bonferoni, C.; Scarpellini, A.; Brunetti, V.; Carzino, R.; Bianchini, P.; Pompa, P.P.; Athanassiou, A. All natural cellulose acetate—Lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M. Essential oils and nanotechnology for combating microbial biofilms. Curr. Org. Chem. 2013, 17, 90–96. [Google Scholar] [CrossRef]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.M.; Grumezescu, A.M.; Saviuc, C.; Croitoru, C.; Mihaiescu, D.E.; Lazar, V. Improved antibacterial activity of cephalosporins loaded in magnetic chitosan microspheres. Int. J. Pharm. 2012, 436, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sanpo, N.; Berndt, C.C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cui, H.; Zeng, G.; Chen, M.; Zhang, H.; Xu, M.; Shen, X.; Bortolini, C.; Dong, M. Ag-CuFe2O4 magnetic hollow fibers for recyclable antibacterial materials. J. Mater. Chem. B 2013, 1, 2719–2723. [Google Scholar] [CrossRef]
- Ahmed, T.T.; Rahman, I.Z.; Rahman, M.A. Study on the properties of the copper substituted NiZn ferrites. J. Mater. Process. Technol. 2004, 153–154, 797–803. [Google Scholar] [CrossRef]
- Dötsch, H.; Tolksdorf, W.; Welz, F. Domain-wall oscillation of bubble and stripe lattices in hexagonal ferrites. J. Appl. Phys. 1980, 51, 3816–3820. [Google Scholar] [CrossRef]
- Nayak, P.K. Synthesis and characterization of cadmium ferrite. Mater. Chem. Phys. 2008, 112, 24–26. [Google Scholar] [CrossRef]
- Kumar, G.R.; Kumar, K.V.; Venudhar, Y.C. Synthesis, structural and magnetic properties of copper substituted nickel ferrites by sol-gel method. Mater. Sci. Appl. 2012, 3, 87–91. [Google Scholar] [CrossRef]
- Sanpo, N.; Berndt, C.C.; Wang, J. Microstructural and antibacterial properties of zinc-substituted cobalt ferrite nanopowders synthesized by sol-gel methods. J. Appl. Phys. 2012, 112. [Google Scholar] [CrossRef]
- Srinivas, B.T.V.; Rawat, V.S.; Konda, K.; Sreedhar, B. Magnetically separable copper ferrite nanoparticles-catalyzed synthesis of diaryl, alkyl/aryl sulfones from arylsulfinic acid salts and organohalides/boronic acids. Adv. Synth. Catal. 2014, 356, 805–817. [Google Scholar] [CrossRef]
- Heinrich, D.; Goni, A.R.; Osan, T.M.; Cerioni, L.M.C.; Smessaert, A.; Klapp, S.H.L.; Faraudo, J.; Pusiol, D.J.; Thomsen, C. Effects of magnetic field gradients on the aggregation dynamics of colloidal magnetic nanoparticles. Soft Matter 2015, 11, 7606–7616. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Steelandt, J.; Salmon, D.; Gilbert, E.; Almouazen, E.; Renaud, F.N.R.; Roussel, L.; Haftek, M.; Pirot, F. Antimicrobial nanocapsules: From new solvent-free process to in vitro efficiency. Int. J. Nanomed. 2014, 9, 4467–4474. [Google Scholar]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef]
- Schulz, H. Rapid analysis of medicinal and aromatic plants by non-destructive vibrational spectroscopy methods. Acta Hort. 2005, 679, 181–187. [Google Scholar] [CrossRef]
- McKenna, F.E.; Tartar, H.V.; Lingafelter, E.C. Studies of hemiacetal formation in alcohol—Aldehyde systems. II. Refraction studies1. J. Am. Chem. Soc. 1953, 75, 604–607. [Google Scholar] [CrossRef]
- Azofra, L.M.; Alkorta, I.; Elguero, J.; Toro-Labbé, A. Mechanisms of formation of hemiacetals: Intrinsic reactivity analysis. J. Phys. Chem. A 2012, 116, 8250–8259. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, B.; Sitarz, M.; Olejnik, E.; Kaczmarska, K. FT-IR and FT-Raman studies of cross-linking processes with Ca2+ ions, glutaraldehyde and microwave radiation for polymer composition of poly(acrylic acid)/sodium salt of carboxymethyl starch—Part I. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bilcu, M.; Grumezescu, A.; Oprea, A.; Popescu, R.; Mogoșanu, G.; Hristu, R.; Stanciu, G.; Mihailescu, D.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of vanilla, patchouli and ylang ylang essential oils stabilized by iron oxide@C14 nanostructures against bacterial adherence and biofilms formed by Staphylococcus aureus and klebsiella pneumoniae clinical strains. Molecules 2014, 19, 17943. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.; Holban, A.; Ficai, A.; Anghel, A.; Chifiriuc, M. Biohybrid nanostructured iron oxide nanoparticles and satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.-W.; Choi, S.; Noh, W.-T.; Kwon, J.; Cho, J.Y.; Chae, D.-Y.; Kim, K.-S. Preparation of iron nanoparticles in cellulose acetate polymer and their reaction chemistry in the polymer. Bull. Korean Chem. Soc. 2001, 22, 772–774. [Google Scholar]
- Shim, I.-W.; Noh, W.-T.; Kwon, J.; Cho, J.Y.; Kim, K.-S.; Kang, D.H. Preparation of copper nanoparticles in cellulose acetate polymer and the reaction chemistry of copper complexes in the polymer. Bull. Korean Chem. Soc. 2002, 23, 563–566. [Google Scholar]
- Sheny, D.S.; Mathew, J.; Philip, D. Synthesis characterization and catalytic action of hexagonal gold nanoparticles using essential oils extracted from anacardium occidentale. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Xia, M.-S. Adsorption and antibacterial effect of copper-exchanged montmorillonite on Escherichia coli K88. Appl. Clay Sci. 2006, 31, 180–184. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds Cu-ferrite NPs, CA/5-LG NCs, CA/5-LG/Cu-ferrite NCs are available from the authors.










© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakos, I.L.; Abdellatif, M.H.; Innocenti, C.; Scarpellini, A.; Carzino, R.; Brunetti, V.; Marras, S.; Brescia, R.; Drago, F.; Pompa, P.P. Antimicrobial Lemongrass Essential Oil—Copper Ferrite Cellulose Acetate Nanocapsules. Molecules 2016, 21, 520. https://doi.org/10.3390/molecules21040520
Liakos IL, Abdellatif MH, Innocenti C, Scarpellini A, Carzino R, Brunetti V, Marras S, Brescia R, Drago F, Pompa PP. Antimicrobial Lemongrass Essential Oil—Copper Ferrite Cellulose Acetate Nanocapsules. Molecules. 2016; 21(4):520. https://doi.org/10.3390/molecules21040520
Chicago/Turabian StyleLiakos, Ioannis L., Mohamed H. Abdellatif, Claudia Innocenti, Alice Scarpellini, Riccardo Carzino, Virgilio Brunetti, Sergio Marras, Rosaria Brescia, Filippo Drago, and Pier Paolo Pompa. 2016. "Antimicrobial Lemongrass Essential Oil—Copper Ferrite Cellulose Acetate Nanocapsules" Molecules 21, no. 4: 520. https://doi.org/10.3390/molecules21040520