Trace Element Distribution in Magnetite Separates of Varying Origin: Genetic and Exploration Significance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Chemistry
Whole Rock Analysis
3. Geological Outline and Mineralogical Features
3.1. Hydrothermal Magnetite Separates
3.1.1. Massive Magnetite Separates of Skarn-Type (Lavrion, Plaka Mine)
3.1.2. Disseminated Magnetite in Porphyry-Cu Deposit (Skouries)
3.1.3. Massive Magnetite Associated with Fe–Cu–Ni–Co Sulphides in Ophiolites (Pindos)
3.1.4. Massive Magnetite in Mafic Ophiolitic Rocks Edessa (Samari)
3.1.5. Massive Magnetite Associated with Apatite in Ophiolites
3.2. Magmatic Magnetite Separates
3.2.1. Disseminated Magnetite in Ultramafic Lavas and Wherlites
3.2.2. Disseminated Magnetite Separates in Norite Gabbros (Central Vourinos, Krapa Hills)
3.3. Magnetite in Coastal Black Sand
3.4. Magnetite Separates from Metamorphosed Fe–Ni-Laterites (Olympos, Edessa, and Vermion)
4. Geochemical Characteristics of Magnetite Separates
5. Discussion
5.1. Trace Element Distribution in Magnetite Structure and Associated Minerals
5.2. Re-Mobilization and Re-Deposition of Magnetite
5.3. Implications of the Trace Element Distribution for the Origin of Magnetite Mineralization
5.4. Implications of Magnetite Separates for Exploration of Precious Metals in Porphyry-Cu Deposits
6. Conclusions
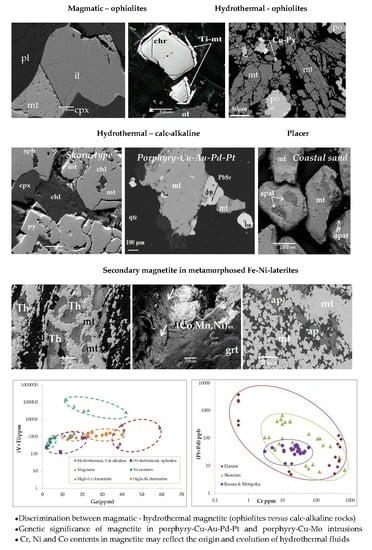
- Magnetite separates of hydrothermal origin associated with calc-alkaline rocks can be distinguished from that associated with ophiolites in terms of their higher Ga content, while magnetite separates of magmatic origin are characterized by the highest (V + Ti) content.
- Hydrothermal magnetite separates from skarn-type (Lavrion mine) differs compared to the disseminated separates from porphyry-Cu deposit (Skouries) in terms of the higher Ba, Bi, Cr, Ni, Co, V, Sr, Sc, Rb, ΣREE, Y, Hf, Th, U, and Zr contents in the latter.
- Although an initial primary magmatic origin for the Fe–Cu–Ni–Co sulphides in the Pindos ophiolite is not precluded, geochemical characteristics (REE-, Ti-, and V-depleted) of the highly transformed magnetite separates may indicate that potential original magmatic features have been overprinted by low-level hydrothermal circulation processes.
- Similarity between the REE patterns, including the clear Eu negative anomaly of magnetite separates from coastal black sand of Kos Island, may provide evidence of an andesitic source.
- A major requirement controlling the Pd and Pt potential of porphyry-Cu deposits is probably the oxidized nature of parent magmas that facilitate the capacity for transporting sufficient Pd and Pt, and the crystallization of abundant magnetite.
- Abundance of magnetite in porphyry-type deposits may be used to discriminate between porphyry-Cu–Au–Pd–Pt and porphyry-Cu–Mo deposits lacking precious metals, providing evidence for the exploration of precious metals in porphyry systems.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paraskevopoulos, G.M.; Economou, M.I. Zoned Mn-rich chromite from podiform type chromite ore in serpentinites of northern Greece. Am. Miner. 1981, 66, 1013–1019. [Google Scholar]
- Economou, M.; Skounakis, S.; Papathanasiou, C. Magnetite deposits of skarn type from the Plaka area of Laurium. Greece 1981, 40, 241–252. [Google Scholar]
- Lindsley, D.H. Experimental studies of oxide minerals: Reviews Mineral. Geochemistry 1991, 25, 69–106. [Google Scholar]
- Eliopoulos, D.G.; Economou-Eliopoulos, M. Platinum-group element and gold contents in the Skouries porphyry-copper deposit, Chalkidiki Peninsula, northern Greece. Econ. Geol. 1991, 86, 740–749. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmoch. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Hayes, T.S.; Koenig, A.E.; Box, S.E. Geochemistry of magnetite from hydrothermal ore deposits and host rocks of the Mesoproterozoic Belt Supergroup, United States. Econ. Geol. 2012, 107, 1275–1292. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore. Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G.; Mιric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2012, 49, 785–796. [Google Scholar] [CrossRef]
- Rapp, J.F.; Klemme, S.; Butler, I.B.; Harley, S.L. Extremely high solubility of rutile in chloride and fluoride-bearing metamorphic fluids: An experimental investigation. Geology 2010, 38, 323–326. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M. Apatite and Mn, Zn, Co-enriched chromite in Ni-laterites of northern Greece and their genetic significance. J. Geochem. Exp. 2003, 80, 41–54. [Google Scholar] [CrossRef]
- Augé, T.; Petrunov, R.; Bailly, L. On the mineralization of the PGE mineralization in the Elastite porphyry Cu–Au deposit, Bulgaria: Comparison with the Baula-Nuasahi Complex, India, and other alkaline PGE-rich porphyries. Can. Miner. 2005, 43, 1355–1372. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M. Platinum-Group Element Potential of Porphyry Deposits. In Mineralogical Association of Canada Short Course 35; Mineralogical Association of Canada: Quebec, QC, Canada, 2005; pp. 203–245. [Google Scholar]
- Navrotsky, A.; Kleppa, O.J. The thermodynamics of cation distributions in simple spinels. J. Inorg. Nucl. Chem. 1967, 29, 2701–2714. [Google Scholar] [CrossRef]
- O’Neill, H.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Miner. 1983, 68, 181–194. [Google Scholar]
- Hu, H.; Lentz, D.; Li, J.W.; McCarron, T.; Zhao, X.F.; Hall, D. Reequilibration processes in magnetite from iron skarn deposits. Econ. Geol. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Marinos, G.P.; Petrascheck, W.E. Laurium. Geol. Geophys. Res. 1956, 4, 1–247. [Google Scholar]
- Skarpelis, N.; Tsikouris, B.; Pe-Piper, G. The Miocene igneous rocks in the Basal Unit of Lavrion (SE Attica, Greece): Petrology and geodynamic implications. Geol. Mag. 2007, 145, 1–15. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.; Bonsall, T.; Tarkian, M.; Economou-Eliopoulos, M. Mineralogical and fluid inclusion constraints on the evolution of the Plaka intrusion-related ore system, Lavrion, Greece. Miner. Petrol. 2008, 93, 79–110. [Google Scholar] [CrossRef]
- Ducoux, M.; Branquet, Y.; Jolivet, L.; Arbaret, L.; Grasemann, B.; Rabillard, A.; Gumiaux, C.; Drufin, S. Synkinematic skarns and fluid drainage along detachments: The West Cycladic Detachment System on Serifos Island (Cyclades, Greece) and its related mineralization. Tectonophysics 2017, 695, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kockel, F.; Mollat, H.; Gundlach, H. Hydrothermally altered and (copper) mineralizedp orphyritic intrusionsin the ServomacedoniaMn assif s (Greece). Miner. Depos. 1975, 10, 195–204. [Google Scholar] [CrossRef]
- Pearce, J.A.; Lippard, S.J.; Roberts, S. Characteristics and tectonic significance of supra-subduction zone ophiolites. Geol. Soc. Lond. Spec. Pub. 1984, 16, 77–94. [Google Scholar] [CrossRef]
- Saccani, E.; Photiades, A.; Santato, A.; Zeda, O. New evidence for supra-subduction zone ophiolites in the Vardar Zone from the Vermion Massif (northern Greece): Implication for the tectono-magmatic evolution of the Vardar oceanic basin. Ofioliti 2008, 33, 17–37. [Google Scholar]
- Economou-Eliopoulos, M.; Eliopoulos, D.; Chryssoulis, S. A comparison of high-Au massive sulfide ores hosted in ophiolite complexes of the Balkan Peninsula with modern analogues: Genetic significance. Ore Geol. Rev. 2008, 33, 81–100. [Google Scholar] [CrossRef]
- Paraskevopoulos, G.M.; Economou, M.I. Genesis of magnetite ore occurrences by metasomatism of chromite ores in Greece. Neues Jahrb. Fur Miner. Abh. 1980, 140, 29–53. [Google Scholar]
- Mercier, J. Édute géologique des zones internes des Hellénides en Macédoine centrale (Grèce). Ire Thèse (1966). Ann. Géol. Des. Pays Hell. 1968, 20, 596. [Google Scholar]
- Sideris, C.; Skounakis, S.; Economou, M. The ophiolite complex of Edessa area and the associated mineralization. International Symposium on metallogeny of mafic and ultramafic complexes. I.G.C.P. 169 Athens 1980, 2, 142–153. [Google Scholar]
- Hynes, A.J. The Geology of Part of the Western Othrys Mountains, Greece. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1972. [Google Scholar]
- Rassios, A.; Dilek, Y. Rotational deformation in the Jurassic Mesohellenic Ophiolites, Greece, and its tectonic significance. Lithos 2009, 108, 207–223. [Google Scholar] [CrossRef]
- Rassios, A.; Konstantopoulou, G. Emplacement tectonism and the position of chrome ores in the Mega Isoma peridotites, SW Othris, Greece. Bull. Geol. Soc. Greece 1993, 28, 463–474. [Google Scholar]
- Mitsis, I.; Economou-Eliopoulos, M. Occurrence of apatite with magnetite in an ophiolite complex (Othrys), Greece. Am. Miner. 2001, 86, 1143–1150. [Google Scholar] [CrossRef]
- Paraskevopoulos, G.; Economou, M. Komatiite-type ultramafic lavas from the Agrilia Formation, Othrys ophiolite complex, Greece. Ofioliti 1986, 11, 293–304. [Google Scholar]
- Economou-Eliopoulos, M.; Paraskevopoulos, G. Platinum -group elements and gold in komatiitic rocks from the Agrilia Formation, Othrys ophiolite complex, Greece. Chem. Geol. 1989, 77, 149–158. [Google Scholar] [CrossRef]
- Koutsovitis, P.; Magganas, A.; Ntaflos, T. Rift and intra-oceanic subduction signatures in the Western Tethys during the Triassic: The case of ultramafic lavas as part of an unusual ultramafic–mafic–felsic suite in Othris, Greece. Lithos 2012, 144, 177–193. [Google Scholar] [CrossRef]
- Baziotis, I.; Economou-Eliopoulos, M.; Asimow, P.D. Ultramafic lavas and high-Mg basaltic dykes from the Othris ophiolite complex, Greece. Lithos 2017, 288, 231–247. [Google Scholar] [CrossRef] [Green Version]
- Rassios, A. Geology and Evolution of the Vourinos Complex, Northern Greece. Ph.D. Thesis, University of California (Davis), Davis, CA, USA, 1981. [Google Scholar]
- Francalanci, L.; Varekamp, J.C.; Vougioukalakis, G.; Defant, M.J.; Innocenti, F.; Manetti, P. Crystal retention, fractionation and crustal assimilation in aconvecting magma chamber Nisyros Volcano, Greece. Bull. Volcanol. 1995, 56, 601–620. [Google Scholar] [CrossRef]
- Papanikolaou, D. Geotectonic evolution of the Aegean. Bull. Geol. Soc. Greece 1993, 27, 33–48. [Google Scholar]
- Royden, L.H.; Papanikolaou, D.J. Slab segmentation and late Cenozoicdisruption of the Hellenic arc. Geochem. Geophys. Geosyst. 2011, 12, Q03010. [Google Scholar] [CrossRef]
- Mitropoulos, P.; Tarney, J.; Saunders, A.D.; Marsh, N.G. Petrogenesis of Cenozoic rocks from the aegean island arc. J. Volcanol. Geotherm. Res. 1987, 32, 177–193. [Google Scholar] [CrossRef]
- Tzifas, I.T.; Misaelides, P.; Godelitsas, A.; Gamaletsos, P.N.; Nomikou, P.; Karydas, A.G.; Kantarelou, V.; Papadopoulos, A. Geochemistry of coastal sands of Eastern Mediterranean: The case of Nisyros volcanic materials. Chem. Der Erde-Geochem. 2017, 77, 487–501. [Google Scholar] [CrossRef]
- Marcopoulos, T.; Economou, M. Theophrastite, Ni(OH)2, a new mineral from Northern Greece. Am. Miner. 1981, 66, 1020–1021. [Google Scholar]
- Economou-Eliopoulos, M.; Eliopoulos, D. A New Solid Solution [(Co,Mn,Nn)(OH)2], in the Vermion Mt (Greece) and its Genetic Significance for the Mineral Group of Hydroxides; McLaughlin, E.D., Braux, L.A., Eds.; Chemical Mineralogy, Smelting and Metallization; Nova Science Publishers: New York, NY, USA, 2009; pp. 1–18. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific: Oxford, UK, 1985. [Google Scholar]
- Gamaletsos, P. Mineralogy and Geochemistry of Bauxites from Parnassos-Ghiona Mines and the Impact on the Origin of the Deposits. Ph.D. Thesis, University of Athens, Athens, Greece, 2014. [Google Scholar]
- Kalatha, S. Metallogenesis of Bauxite Laterites and Fe-Ni-laterites-Enrichnment in Rare Earth Elements. Ph.D. Thesis, University of Athens, Athens, Greece, 2017. [Google Scholar]
- Barth, M.; Gluhak, T. Geochemistry and tectonic setting of mafic rocks from the Othris Ophiolite, Greece. Contrib. Miner. Petrol. 2009, 157, 23–40. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. Chemical evolution of the mantle. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Sotnikov, V.I.; Berzina, A.N.; Economou-Eliopoulos, M.; Eliopoulos, D.G. Palladium, platinum and gold distribution in porphyry Cu _ Mo deposits of Russia and Mongolia. Ore Geol. Rev. 2001, 18, 95–111. [Google Scholar] [CrossRef]
- Schock, H.H. Distribution of rare-earth and other trace elements in magnetite separates. Chem. Geol. 1979, 26, 119–133. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contrib. Miner. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Horn, I.; Foley, S.F.; Jackson, S.E.; Jenner, G.A. Experimental determined partitioning of high field strength and selected transition elements between spinel and basaltic melt. Chem. Geol. 1994, 117, 193–318. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Maksimovic, Z.; Skarpelis, N.; Panto, G. Mineralogy and geochemistry of the rare earth elements in the karstic nickel deposit of Lokris area, Greece. Acta Geol. Hung. 1993, 36, 331–342. [Google Scholar]
- Economou-Eliopoulos, M.; Eliopoulos, D.G.; Apostolikas, A.; Maglaras, K. Precious and rare earth element distribution in Ni-laterite deposits from Lokris area, Central Greece. In Proceedings of the Fourth Biennial SGA Meeting, Turku, Finland, 11–13 August 1997; pp. 411–413. [Google Scholar]
- Kalatha, S.; Perraki, M.; Economou-Eliopoulos, M.; Mitsis, I. On the origin of bastnaesite-(La,Nd,Y) in the Nissi (Patitira) bauxite laterite deposit, Lokris, Greece. Minerals 2017, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Muller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Colony, W.E.; Nordlie, B.E. Liquid sulfur at Volcan Azufre, Galapagos Islands. Econ. Geol. 1973, 68, 371–380. [Google Scholar] [CrossRef]
- GustafsonL, B.; Hunt, J.P. The porphyry copper deposit at E1 Salvador, Chile. Econ. Geol. 1975, 70, 857–912. [Google Scholar] [CrossRef]
- Hemley, J.J.; Hunt, J.P. Hydrothermal ore-forming processes in the light of studies in rock-buffered systems. II. Some general geologic applications. Econ. Geol. 1992, 87, 23–43. [Google Scholar]
- Hemley, J.J.; Cygan, G.L.; Fein, J.B.; Robinson, G.R.; d’Angelo, W.M. Hydrothermal ore-forming processes in the light of studies in rock-buffered systems I.: Iron-copper-zinc-lead sulfide solubility relations. Econ. Geol. 1992, 87, 1–22. [Google Scholar] [CrossRef]
- Bliss, N.W.; MacLean, W.H. The paragenesis of zoned chromite from central Manitoba. Geochim. Cosmochim. Acta 1975, 39, 973–990. [Google Scholar] [CrossRef]
- Bosi, F.; Andreozzi, G.B.; Hålenius, U.; Skogby, H. Zn-O tetrahedral bond length variations in normal spinel oxides. Am. Mineral. 2011, 96, 594–598. [Google Scholar] [CrossRef]
- Martignago, F.; Dal Negro, A.; Carbonin, S. How Cr3+ and Fe3+ affect Mg-Al order disorder transformation at high temperature in natural spinels. Phys. Chem. Miner. 2003, 30, 401–408. [Google Scholar] [CrossRef]
- Gervilla, F.; Padrón-Navarta, J.A.; Kerestedjian, T.; Sergeeva, I.; González-Jiménez, J.M.; Fanlo, I. Formation of ferrian chromite in podiform chromitites from the Golyamo Kamenyane serpentinte, Eastern Rhodopes,SE Bulgaria: A two-stage process. Contrib. Mineral. Petrol. 2012, 164, 643–657. [Google Scholar] [CrossRef]
- Colás, V.; González-Jiménez, J.M.; Griffin, W.L.; Fanlo, I.; Gervilla, F.; O’Reilly, S.Y.; Pearson, N.J.; Kerestedjian, T.; Proenza, J.A. Fingerprints of metamorphism in chromite: New insights from minor and trace elements. Chem. Geol. 2014, 389, 137–152. [Google Scholar] [CrossRef]
- Glemser, O.; Einerhand, J. Uber hohere Nickelhydroxide. Z. Anorg. Chem. 1950, 261, 26–42. [Google Scholar] [CrossRef]
- Baskar, S.; Baskar, R.; Kaushik, A. Role of microorganisms in weathering of the Konkan-Goa laterite formation. Curr. Sci. 2003, 85, 1129–1134. [Google Scholar]
- Russell, M.J.; Hall, A.J.; Boyce, A.J.; Fallick, A.E. On hydrothermal convection systems and the emergence of life. Econ. Geol. 2005, 100, 418–438. [Google Scholar]
- Southam, G.; Saunders, J. The geomicrobiology of ore deposits. Econ. Geol. 2005, 100, 1067–1084. [Google Scholar] [CrossRef]
- Valeton, I.; Biermann, M.; Reche, R.; Rosenberg, F. Genesis of Nickel laterites and bauxites in Greece during the Jurassic and Cretaceous, and their relation to ultrabasic parent rocks. Ore Geol. Rev. 1987, 2, 359–404. [Google Scholar] [CrossRef]
- Eliopoulos, D.; Economou-Eliopoulos, M. Geochemical and mineralogical characteristics of Fe–Ni and bauxite–laterite deposits of Greece. Ore Geol. Rev. 2000, 16, 41–58. [Google Scholar] [CrossRef]
- Kalatha, S.; Economou-Eliopoulos, M. Framboidal pyrite and bacteriomorphic goethite at transitional zones between Fe–Ni–laterites and limestones: Evidence from Lokris, Greece. Ore Geol. Rev. 2015, 65, 413–425. [Google Scholar] [CrossRef]
- Norton, S. Laterite and bauxite formation. Econ. Geol. 1973, 63, 353–361. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. Bio-mineralization and potential biogeochemical processes in bauxite deposits: Genetic and ore quality significance. Miner. Petrol. 2013, 107, 471–486. [Google Scholar] [CrossRef]
- Schulte, R.F.; Foley, N.K. Compilation of Gallium Resource Data for Bauxite Deposits; U.S. Geological Survey: Reston, VA, USA, 2014.
- Zhou, M.F.; Robinson, P.T.; Su, B.X.; Gao, J.F.; Li, J.W.; Yang, J.S.; Malpas, J. Compositions of chromite, associated minerals, and parental magmas of podiform chromite deposits: The role of slab contamination of asthenospheric melts in suprasubduction zone environments. Gondwana Res. 2014, 26, 262–283. [Google Scholar] [CrossRef]
- Eliopoulos, I.P.D.; Eliopoulos, G.D. Factors Controlling the Gallium Preference in High-Al Chromitites. Minerals 2019, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Tarkian, M.; Koopmann, G. Platinum-group minerals in the Santo Tomas II (Philex) porphyry copper–gold deposit, Luzon Island, Philippines. Miner. Depos. 1995, 30, 39–47. [Google Scholar] [CrossRef]
- Thompson, J.F.H.; Lang, J.R.; Stanley, C.R. Platinum-group elements in alkaline porphyry deposits, British Columbia. Explor. Min. B. C. Mines Branch Part B 2001, 2001, 57–64. [Google Scholar]
- Richards, J.P. Tectono-magmatic precursors for porphyry Cu–(Mo–Au) deposit formation. Econ. Geol. 2003, 98, 1515–1533. [Google Scholar] [CrossRef]
- Richards, J.P. Postsubduction porphyry Cu–Au and epithermal Au deposits: Products of remelting of subduction-modified lithosphere. Geology 2009, 37, 247–250. [Google Scholar] [CrossRef]
- Richards, J. Tectonic, magmatic, metallogenic evolution of the Tethyan orogeny: From subduction to collision. Ore Geol. Rev. 2015, 70, 323–345. [Google Scholar] [CrossRef]
- Tarkian, M.; Hünken, U.; Tokmakchieva, M.; Bogdanov, K. Precious-metal distribution and fluid-inclusion petrography of the Elatsite porphyry copper deposit, Bulgaria. Miner. Depos. 2003, 38, 261–281. [Google Scholar] [CrossRef]
- Eliopoulos, D.G.; Economou-Eliopoulos, M.; Zelyaskova-Panayiotova, M. Critical factors controlling Pd and Pt potential in porphyry Cu–Au deposits: Evidence from the Balkan Peninsula. Geosciences 2014, 4, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Holwell, D.; Fiorentini, M.; McDonald, I.; Lu, Y.; Giuliani, A.; Smith, D.; Keith, M.; Locmelis, M. A metasomatized lithospheric mantle control on the metallogenic signature of post-subduction magmatism. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mungall, J.E.; Andrews, D.R.A.; Cabri, L.J.; Sylvester, P.J.; Tubrett, M. Partitioning of Cu, Ni, Au, and platinum-group elements between monosulfide solid solution and sulfide melt under controlled oxygen and sulphur fugacities. Geochim. Cosmochim. Acta 2005, 69, 4349–4360. [Google Scholar] [CrossRef] [Green Version]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Berzina, A.P.; Berzina, A.N.; Gimon, V.O. Paleozoic–Mesozoic porphyry Cu (Mo) and Mo (Cu) deposits within the southern margin of the Siberian Craton: Geochemistry, geochronology, and petrogenesis (a review). Minerals 2016, 6, 125. [Google Scholar] [CrossRef] [Green Version]
- McInnes, B.I.A.; Cameron, E.M. Carbonated, alkaline hybridizing melts from a sub-arc environment: Mantle wedge samples from the Tabar-Lihir-Tanga-Feni arc, Papua New Guinea. Earth Planet. Sci. Lett. 1994, 122, 125–141. [Google Scholar] [CrossRef]
- Moritz, R.; Kouzmanov, K.; Petrunov, R. Upper Cretaceous Cu–Au epithermal deposits of the Panagyurishte district, Srednogorie zone, Bulgaria. Swiss Bull. Miner. Petrol. 2004, 84, 79–99. [Google Scholar]














| CALC-ALKALINE | OPHIOLITES | PLACER | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deposit type | Skarn-type | Hydrothermal | Hydrothermal | Magmatic | |||||||||
| Location | Lavrion | Skouries | Othrys | Edessa | Pindos | Vourinos | Vourinos | Othrys | Othrys | KOS | |||
| Plaka | Plaka | Agoriani | Samari | Perivoli | Krapa | Krapa | Agrilia | Agrilia | |||||
| sample | P.L.mt1 | P.L.mt2 | SK43 | SG-6 | Skou8 | O.Ag.t | EdK2 | Pi.Mt | VKMt | VKMt1 | 15Agr2 | O.Agr34 | KOS1 |
| wt.% | |||||||||||||
| SiO2 | 1.7 | 2.6 | 0.9 | 0.7 | 0.4 | 0.5 | 2.3 | 0.3 | 0.5 | 0.9 | 0.3 | 0.7 | 0.5 |
| Al2O3 | 0.5 | 0.9 | n.d | n.d. | n.d. | 0.6 | 0.2 | n.d. | 1.7 | 2.1 | 11.1 | 7.5 | 1.5 |
| Cr2O3 | n.d. | n.d. | 2.1 | 0.9 | n.d. | n.d. | 0.1 | n.d. | n.d. | n.d. | 3.9 | 1.5 | 0.2 |
| V2O5 | n.d. | n.d. | 0.7 | 0.5 | n.d. | n.d. | n.d. | n.d. | 1.4 | 1.8 | 0.7 | 0.6 | 0.5 |
| Fe2O3 | 67.5 | 67.7 | 66.7 | 67.6 | 67.9 | 68.1 | 68.1 | 69.7 | 55.1 | 55.4 | 45.2 | 51.4 | 57.1 |
| FeO | 30.4 | 30.4 | 30.6 | 30.8 | 30.7 | 30.1 | 29.9 | 30.2 | 33.5 | 33.3 | 32.3 | 31.1 | 34.5 |
| MgO | n.d. | 0.3 | n.d. | n.d. | 0.5 | 0.9 | n.d. | 0.2 | n.d. | 0.9 | 3.1 | 3.5 | n.d. |
| MnO | 0.5 | 0.3 | n.d. | n.d. | n.d. | n.d. | 0.2 | 0.2 | 0.5 | 0.6 | 0.2 | 0.4 | 1.2 |
| CoO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.2 | n.d. | n.d. |
| TiO2 | n.d. | n.d. | n.d. | n.d. | 0.6 | n.d. | n.d. | n.d. | 6.9 | 4.4 | 3.6 | 4.6 | 5.1 |
| Total | 100.6 | 101.5 | 101 | 99.6 | 99.8 | 99.3 | 100.8 | 100.6 | 99.6 | 99.4 | 100.9 | 100.7 | 100.6 |
| wt.% | Monazite | Thorite | U-Thorite | Na, Ce-epidote | Allanite | Allanite |
|---|---|---|---|---|---|---|
| SiO2 | 1.1 | 18.8 | 15.7 | 38.3 | 32.1 | 35.4 |
| Al2O3 | n.d. | n.d. | n.d. | 27.2 | 16.2 | 14.1 |
| FeO | n.d. | n.d. | 0.6 | 8.3 | 14.9 | 17.4 |
| CaO | 0.8 | n.d. | n.d. | 22.2 | 12.4 | 13.6 |
| P2O5 | 30.3 | n.d. | n.d. | n.d. | n.d. | n.d. |
| ZrO2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| La2O3 | 17.2 | n.d. | n.d. | 0.9 | 5.1 | 3.9 |
| Ce2O3 | 31.6 | n.d. | n.d. | 2.2 | 14.5 | 12.5 |
| Nd2O3 | 10.5 | n.d. | n.d. | n.d. | 4.7 | 2.2 |
| Pr2O3 | 2.8 | n.d. | n.d. | n.d. | n.d. | n.d. |
| ThO2 | 5.1 | 79.4 | 70.8 | n.d. | n.d. | n.d. |
| UO3 | n.d. | n.d. | 11.7 | n.d. | n.d. | n.d. |
| Total | 99.4 | 98.2 | 98.1 | 99.1 | 99.9 | 99.1 |
| Host | Devitrified Glass (d.G.) | |||||||
|---|---|---|---|---|---|---|---|---|
| Rhonite (Rho) | d.G. | Amphibole (amph) | ||||||
| SiO2 | 26.3 | 26.6 | 25.8 | 33.1 | 45.6 | 39.8 | 40.4 | 55.2 |
| MgO | 8.8 | 8.7 | 8.8 | 14.5 | 6.5 | 9.2 | 10.6 | 11.1 |
| Al2O3 | 15.7 | 14.9 | 15.2 | 12.1 | 9.9 | 16.5 | 17.5 | 10.6 |
| Cr2O3 | n.d. | n.d. | n.d. | 0.8 | 1.1 | n.d. | n.d. | 1.3 |
| CaO | 10.7 | 10.6 | 10.6 | 4.3 | 3.1 | 10.1 | 9.6 | 10.4 |
| TiO2 | 4.9 | 3.9 | 4.7 | 1.6 | 0.2 | 1.9 | 1.2 | 0.2 |
| FeO | 28.9 | 29.8 | 30.3 | 27.1 | 21.8 | 17.7 | 17.1 | 6.2 |
| MnO | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | n.d. | 0.3 | n.d. |
| Na2O | 0.5 | 0.5 | 1.3 | 1.6 | 6.9 | 2.5 | 1.7 | 2.7 |
| K2O | n.d. | n.d. | n.d. | 0.2 | 0.9 | 0.3 | 0.2 | 0.3 |
| NiO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cl | n.d. | n.d. | n.d. | n.d. | n.d. | 0.2 | 0.2 | n.d. |
| Total | 96.0 | 95.2 | 97.0 | 95.5 | 96.2 | 98.2 | 98.8 | 98.0 |
| Olympos | Edessa | E. Vermion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Paliambela | Nissi | Mavrolivado | Stournari | |||||||||
| Core | Rim | Core | Rim | Core | Rim | ||||||||
| Mineral | Chromite | Fe-chr | mt | Chromite | Fe-chr | mt | Chromite | Fe-chr | mt | Theophastite | (Co,Mn,Ni)-hydroxides | ||
| sample | L1 | L1 | L1 | Ed10 | Ed10 | EdP10 | V.AK | V.AK | V.AK | ||||
| wt.% | |||||||||||||
| SiO2 | 0.2 | n.d. | 0.4 | n.d. | n.d. | 0.5 | 0.3 | 0.7 | 1.2 | n.d. | n.d. | n.d. | n.d. |
| Al2O3 | 8.2 | 8.6 | 0.4 | 9.7 | 6.5 | 0.2 | 24.4 | 7.7 | 0.5 | n.d. | n.d. | n.d. | n.d. |
| Cr2O3 | 62.1 | 53.7 | 5.9 | 60.2 | 58.1 | 1.6 | 43.3 | 42.3 | 1.6 | n.d. | n.d. | n.d. | n.d. |
| Fe2O3 | 1.8 | 3.5 | 62.2 | 2.7 | 3.3 | 67.7 | 2.2 | 17.6 | 66.5 | n.d. | n.d. | n.d. | n.d. |
| FeO | 13.9 | 27.6 | 28.4 | 15.5 | 8.3 | 29.3 | 17.3 | 26.6 | 28.9 | 1.0 | n.d. | n.d. | n.d. |
| MgO | 11.3 | 0.6 | 0.4 | 11.2 | 3.5 | 0.3 | 11.1 | 2.2 | 0.3 | n.d. | n.d. | n.d. | n.d. |
| MnO | 1.9 | 4.1 | 0.5 | 1.6 | 13.1 | n.d. | n.d. | 2.8 | 0.3 | n.d. | 25.0 | 13.9 | 19.2 |
| ZnO | n.d | 0.4 | n.d. | n.d. | 2.1 | n.d. | 0.5 | 0.7 | 0.3 | n.d. | n.d. | n.d. | n.d. |
| CoO | n.d. | n.d. | 0.4 | n.d. | 4.1 | n.d. | n.d. | n.d. | n.d. | n.d. | 38.0 | 54.3 | 46.9 |
| TiO2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n,d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| NiO | n.d | n.d. | 1.6 | n.d. | n.d. | 1.4 | n.d. | 0.2 | n.d. | 79.2 | 17.4 | 11.2 | 13.6 |
| Total | 99.4 | 98.5 | 100.2 | 100.9 | 99.0 | 101 | 99.1 | 100.8 | 99.6 | 80.2 | 80.4 | 79.44 | 79.7 |
| Cr/(Cr + Al) | 0.83 | 0.81 | 0.81 | 0.78 | 0.86 | 0.87 | 0.54 | 0.79 | 0.94 | ||||
| Mg/(Mg + Fe2+) | 0.55 | 0.03 | 0.13 | 0.47 | 0.17 | 0.01 | 0.51 | 0.12 | 0.01 |
| MAGMATIC | Location | Rock-Type | Texture | ΣREE | Ce/Ce* | Eu/Eu* | Pr/Pr* | Gd/Gd* | Ga | V | Ti | Cr | Ni | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ophiolites | Vourinos (Krapa) | Norite gabbro | Disseminated | 6 | 1.06 | 1.43 | 0.92 | 1.14 | 22 | 6520 | 2.6 | 1070 | 100 | Present study |
| Othrys | Ultramafic lava | Disseminated | 132 | 0.9 | 1.97 | 0.98 | 0.65 | 12 | 1820 | 13.4 | 1600 | 160 | Present study | |
| Calc-alkaline | Kos | Coastal sand | Placer | 224 | 0.92 | 0.32 | 0.85 | 1.81 | 42 | 1480 | 2.5 | 1150 | 11 | Present study |
| HYDROTHERMAL | ||||||||||||||
| Ophiolites | Edessa (Samari) | Peridotite | Massive | 10 | 0.94 | 0.11 | 0.95 | 1.75 | 2 | 34 | 0.02 | 560 | 56 | Present study |
| Othrys (Agoriani) | Association with apatite | Massive | 76 | 0.82 | 0.70 | 0.71 | 1.28 | 20 | 640 | 0.02 | 1100 | 280 | Present study | |
| Pindos (Perivoli) | Irregular | Massive | 3.1 | 0.90 | 0.74 | 1.02 | 1.34 | 9 | 80 | <0.01 | 12 | 6 | Present study | |
| Calk-alkaline | Skouries | Porphyry-Cu | Disseminated | 26 | 0.98 | 1.01 | 0.88 | 1.20 | 46 | 760 | 0.25 | 630 | 340 | Present study |
| Lavrion (Plaka) | Skarn | Massive | 3 | 0.81 | 0.48 | 0.94 | 1.64 | 3.9 | 44 | 0.02 | 160 | 30 | Present study | |
| METAMORPHOSED | Olympos,Vermio, Edessa | Ni-laterites (n = 4) | Major, matrix | 37 | 0.41 | 0.32 | 1.45 | 0.93 | 4.2 | 280 | 380 | 33,980 | 9800 | Present study |
| Fe-Ni-laterite | Lokris | Ni-laterites (n = 13) | Rare, matrix | 120 | 1.02 | 0.85 | 0.96 | 1.14 | 7.6 | 75 | 2900 | 1900 | 2200 | [46] |
| Bauxite laterite | Lokris | Bauxitic laterites (n = 6) | Rare, matrix | 350 | 1.01 | 0.71 | 0.97 | 1.2 | 17 | 340 | 7200 | 3500 | 1400 | [46] |
| Bauxite | Parnassos-Giona | Bauxites (n = 17) | Rare, matrix | 470 | 2.64 | 0.69 | 0.58 | 0.8 | 70 | 460 | 16,000 | 820 | 150 | [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliopoulos, D.G.; Economou-Eliopoulos, M. Trace Element Distribution in Magnetite Separates of Varying Origin: Genetic and Exploration Significance. Minerals 2019, 9, 759. https://doi.org/10.3390/min9120759
Eliopoulos DG, Economou-Eliopoulos M. Trace Element Distribution in Magnetite Separates of Varying Origin: Genetic and Exploration Significance. Minerals. 2019; 9(12):759. https://doi.org/10.3390/min9120759
Chicago/Turabian StyleEliopoulos, Demetrios G., and Maria Economou-Eliopoulos. 2019. "Trace Element Distribution in Magnetite Separates of Varying Origin: Genetic and Exploration Significance" Minerals 9, no. 12: 759. https://doi.org/10.3390/min9120759