Evolution of Sulfidic Legacy Mine Tailings: A Review of the Wheal Maid Site, UK
Abstract
:1. Introduction
2. The Wheal Maid Site and Its History
2.1. Site Overview
2.2. Site History
2.3. Historic Processing and Storage of Waste Materials
3. Characterisation of the Wheal Maid Waste
3.1. Waste Types and Mineralogy
3.1.1. Review of Previous Data
3.1.2. Methodology
3.1.3. Results of Additional XRD Analysis
3.1.4. Mine Waste Mineralogy Summary and Discussion
3.2. Geochemical Analysis of Bulk Waste Samples from Wheal Maid
3.2.1. Review of Previous Data
3.2.2. Methodology
3.2.3. Mine Waste Geochemistry Results
3.2.4. Mine Waste Geochemistry Summary and Discussion
3.3. Mine Waste Mineral Chemistry
3.3.1. Methodology
3.3.2. Mineral Chemistry Results
3.3.3. Mine Waste Mineral Chemistry Summary and Discussion
3.4. Mine Waste Bioaccessibility and Leachability
3.4.1. Review of Previous Data
3.4.2. Mine Waste Bioaccessibility and Leachability Summary and Discussion
3.5. Mine Waste Microbiology
3.5.1. Review of Previous Data
3.5.2. Mine Waste Microbiology Summary and Discussion
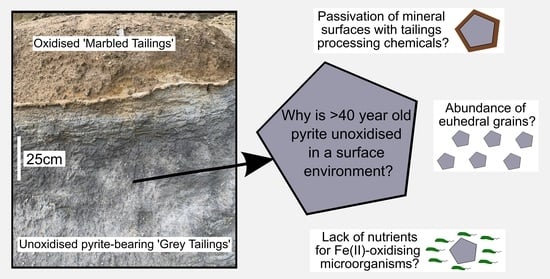
3.6. Investigating the Lack of Oxidation in the Pyritic Grey Tailings
3.6.1. Methodology
- To assess if limited physical contact with atmospheric O2 was contributing to the lack of oxidation, flasks were placed on an orbital shaker (50 rpm), while controls were held under static conditions.
- Nitric acid (0.1 M) was added to investigate whether the pyrite could be oxidised chemically by a relatively weak acid and oxidising agent (in contrast to the strong oxidation reaction of hydrogen peroxide and ammonium acetate used by van Veen et al. 2016). The physical tests above were used as controls.
- To investigate whether a lack of microbial activity was contributing to limited oxidation, flasks were inoculated with a model Fe(II) and S oxidiser, Acidithiobacillus ferrooxidans (grown on pyrite, 10% inoculum added), together with a nutrient solution of 0.5 g L−1 Mg, 0.4 g L−1 NH4+, and 0.2 g L−1 PO42−. The nutrient solution would also stimulate indigenous bacteria in the wastes. Controls for biological oxidation included adding nutrients but no cells and adding cells but no nutrients.
3.6.2. Oxidation Experiment Results
3.6.3. Mine Waste Oxidation Experiment Summary and Discussion
4. Key Data Gaps and Recommendations for Future Research
- Role of microorganisms: Although Fe(II) and sulfur oxidisers have been identified to be present, are they not of sufficient abundance to contribute significantly to pyrite oxidation, particularly Fe(II) and sulfur oxidisers? Or is a lack of nutrients limiting their ability to oxidise pyrite at significant rates?
- Role of processing chemicals: Do the high Na and S concentrations in the Grey Tailings represent the remnants of processing chemicals? Have processing chemicals affected the surface properties of the pyrite (i.e., causing passivation; [34] and so are responsible for limiting its oxidation in the environment?
- Role of pyrite mineralogy: Previous research suggested that the morphology of the pyrite crystals in this particular waste may be inhibiting oxidation. Do the Grey Tailings contain a particularly high proportion of euhedral crystals?
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- URS. Wheal Maid Tailings Lagoon Part IIA Investigation; URS: San Francisco, CA, USA, 2007. [Google Scholar]
- Carrick District Council. Carrick District Council Environmental Protection Act 1990, Part2A—Section 78B Record of Determination of Wheal Maid Tailings Lagoons, Gwennap, Cornwall as Contaminated Land. 2007. Available online: https://www.cornwallhousing.org.uk/media/3625647/2008-09-16-Record-of-Determination.pdf (accessed on 20 June 2021).
- Lottermoser, B. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate-determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Spears, D.A.; Martinez Tarazona, M.R.; Lee, S. Pyrite in UK coals: Its environmental significance. Fuel Coal Util. Environ. 1994, 73, 1051–1055. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Amos, R.T.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, A.I.M. Waste-rock hydrogeology and geochemistry. Appl. Geochem. Environ. Geochem. Mod. Min. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- Jennings, S.R.; Dollhopf, D.J.; Inskeep, W.P. Acid production from sulfide minerals using hydrogen peroxide weathering. Appl. Geochem. 2000, 15, 235–243. [Google Scholar] [CrossRef]
- Craw, D.; Rufaut, C. Geochemical and mineralogical controls on mine tailings rehabilitation and vegetation, Otago Schist, New Zealand. N. Z. J. Geol. Geophys. 2017, 60, 176–187. [Google Scholar] [CrossRef]
- Newsome, L.; Falagán, C. The microbiology of metal mine waste: Bioremediation applications and implications for planetary health. GeoHealth 2021, 5, e2020GH000380. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Met Office. UK Regional Climates. Summaries of the Climate Characteristics of 11 Regions of the UK. 2016. Available online: https://www.metoffice.gov.uk/research/climate/maps-and-data/regional-climates/index (accessed on 13 September 2021).
- Crane, R.A.; Sinnett, D.E.; Cleall, P.J.; Sapsford, D.J. Physicochemical composition of wastes and co-located environmental designations at legacy mine sites in the south west of England and Wales: Implications for their resource potential. Resour. Conserv. Recycl. 2017, 123, 117–134. [Google Scholar] [CrossRef]
- Kettaneh, Y.A.; Badham, J.P.N. Mineralization and paragenesis at the Mount Wellington Mine, Cornwall. Econ. Geol. 1978, 73, 486–495. [Google Scholar] [CrossRef]
- Bowell, R.J.; Rees, S.B.; Barnes, A.; Prestia, A.; Warrender, R.; Dey, B.M. Geochemical assessment of arsenic toxicity in mine site along the proposed Mineral Tramway Project, Camborne, Cornwall. Geochem. Explor. Environ. Anal. 2013, 13, 145–157. [Google Scholar] [CrossRef]
- Jay Mineral Services. Carnon Consolidated Limited, RTZ Metals; Jay Mineral Services: London, UK, 1984. [Google Scholar]
- Down, C.G. Mining in Cornwall Today: An Up-to-Date Review of Cornish Mining; Cornish Chamber of Mines, Mount Wellington: Chacewater, UK, 1985; pp. 17–19. [Google Scholar]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Oelkers, E.; Declercq, J.; Bowell, R. Effects of pH on arsenic mineralogy and stability in Poldice Valley, Cornwall, United Kingdom. Geochemistry 2021, 81, 125798. [Google Scholar] [CrossRef]
- van Veen, E.M.; Lottermoser, B.G.; Parbhakar-Fox, A.; Fox, N.; Hunt, J. A new test for plant bioaccessibility in sulphidic wastes and soils: A case study from the Wheal Maid historic tailings repository in Cornwall, UK. Sci. Total Environ. 2016, 563–564, 835–844. [Google Scholar] [CrossRef]
- CL: AIRE. Development of Category 4 Screening Levels for Assessment of Land Affected by Contamination (No. SP1010). Contaminated Land: Applications in Real Environments. 2014. Available online: https://www.claire.co.uk/projects-and-initiatives/category-4-screening-levels (accessed on 5 May 2022).
- Danyushevsky, L.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.; McGoldrick, P.; Shelley, M. Routine quantitative multi-element analysis of sulphide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Younger, P.L.; Coulton, R.H.; Froggatt, E.C. The contribution of science to risk-based decision-making: Lessons from the development of full-scale treatment measures for acidic mine waters at Wheal Jane, UK. Sci. Total Environ. 2005, 338, 137–154. [Google Scholar] [CrossRef]
- Hunt, L.E.; Howard, A.G. Arsenic speciation and distribution in the Carnon estuary following the acute discharge of contaminated water from a disused mine. Mar. Pollut. Bull. 1994, 28, 33–38. [Google Scholar] [CrossRef]
- Camm, G.S.; Glass, H.J.; Bryce, D.W.; Butcher, A.R. Characterisation of a mining-related arsenic-contaminated site, Cornwall, UK. J. Geochem. Explor. 2004, 82, 1–15. [Google Scholar] [CrossRef]
- Dopson, M.; Johnson, D.B. Biodiversity, metabolism and applications of acidophilic sulfur-metabolizing microorganisms. Environ. Microbiol. 2012, 14, 2620–2631. [Google Scholar] [CrossRef]
- Jones, K. Bioinformatic Analysis of Biotechnologically Important Microbial Communities. Ph.D. Thesis, University of Exeter, Exeter, UK, 2018. [Google Scholar]
- Johnson, D.B.; Bridge, T.A.M. Reduction of ferric iron by acidophilic heterotrophic bacteria: Evidence for constitutive and inducible enzyme systems in Acidiphilium spp. J. Appl. Microbiol. 2002, 92, 315–321. [Google Scholar] [CrossRef]
- Koch, W.F.; Marinenko, G.; Paule, R.C. Development of a standard reference material for rainwater analysis. J. Res. Natl. Bur. Stand. 1986, 91, 33–41. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R.; Phillips, E.J.P. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 1987, 53, 1536–1540. [Google Scholar] [CrossRef] [Green Version]
- Smart, R.S.; Amarantidis, J.; Skinner, W.M.; Prestidge, C.A.; Vanier, L.L.; Grano, S.R. Surface analytical studies of oxidation and collector adsorption in sulfide mineral flotation. In Solid—Liquid Interfaces; Springer: Berlin/Heidelberg, Germany, 2003; pp. 3–62. [Google Scholar]
- Tu, Z.; Wu, Q.; He, H.; Zhou, S.; Liu, J.; He, H.; Liu, C.; Dang, Z.; Reinfelder, J.R. Reduction of acid mine drainage by passivation of pyrite surfaces: A review. Sci. Total Environ. 2022, 832, 155116. [Google Scholar] [CrossRef]
- Helser, J.; Vassilieva, E.; Cappuyns, V. Environment and human health risk assessment of sulfidic mine waste: Bioaccessibility, leaching and mineralogy. J. Hazard. Mater. 2022, 424, 127313. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Abedelmoula, M.; Dynes, J.J.; Jamieson, H. Role of secondary minerals in the acid generating potential of weathered mine tailings: Crystal chemistry characterisation and closed mine site management involvement. Sci. Total Environ. 2021, 784, 147105. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Glass, H.J.; Page, C.N. Sustainable natural remediation of abandoned tailings by metal-excluding heather (Calluna vulgaris) and gorse (Ulex europaeus), Carnon Valley, Cornwall, UK. Ecol. Eng. 2011, 37, 1249–1253. [Google Scholar] [CrossRef]
- Crane, R.A.; Sapsford, D.J. Towards greener lixiviants in value recovery from mine wastes: Efficacy of organic acids for the dissolution of copper and arsenic from legacy mine tailings. Minerals 2018, 8, 383. [Google Scholar] [CrossRef] [Green Version]
- Sapsford, D.J.; Crane, R.A.; Sinnett, D. An exploration of key concepts in application of in situ processes for recovery of resources from high-volume industrial and mine wastes. In Resource Recovery from Wastes: Towards a Circular Economy; Royal Society of Chemistry: London, UK, 2019; pp. 141–167. [Google Scholar]
- Lag-Brotons, A.J.; Velenturf, A.P.; Crane, R.; Head, I.M.; Purnell, P.; Semple, K.T. Resource recovery from waste. Front. Environ. Sci. 2020, 8, 35. [Google Scholar] [CrossRef]





| Material Type | Physical Appearance | Photographs | Location at the Site | Minerals Identified by XRD [20] |
|---|---|---|---|---|
| Marbled Tailings | Visually distinctive red/brown/yellow mottled tailings. Predominantly made up of clay/silt, with sand and some gravel. |  | Located within the lower tailings lagoon. | Major: quartz, tourmaline, albite, and muscovite. Minor: nacrite, clinochlore, and pyrite. |
| Grey Tailings | Distinguishable due to the presence of pale, yellow pyrite minerals, with a grey fine to medium sand matrix. |  | Found in a distinct area within the central western part of the lower lagoon. | Major: quartz, muscovite, tourmaline, and pyrite. Minor: albite, nacrite, and secondary rozenite. |
| Mine Waste | Granular material with varied particle size, but it typically contains clay/silt, sand, and gravel fractions. |  | Found around the perimeter of the tailings depository and was used to construct the dams. | Major: quartz, muscovite, tourmaline, and nacrite. Minor: albite and clinochlore. |
| Capping Material | Found to be granular and contains roughly equal amounts of clay/silt, sand, and gravel material. Although, the gravel fraction visually appeared to be finer and more regularly sorted than the mine waste. |  | Caps underlying tailings across both of the lagoons. | Major: quartz, muscovite, tourmaline, and nacrite. Minor: albite and clinochlore. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitch, V.; Parbhakar-Fox, A.; Crane, R.; Newsome, L. Evolution of Sulfidic Legacy Mine Tailings: A Review of the Wheal Maid Site, UK. Minerals 2022, 12, 848. https://doi.org/10.3390/min12070848
Fitch V, Parbhakar-Fox A, Crane R, Newsome L. Evolution of Sulfidic Legacy Mine Tailings: A Review of the Wheal Maid Site, UK. Minerals. 2022; 12(7):848. https://doi.org/10.3390/min12070848
Chicago/Turabian StyleFitch, Verity, Anita Parbhakar-Fox, Richard Crane, and Laura Newsome. 2022. "Evolution of Sulfidic Legacy Mine Tailings: A Review of the Wheal Maid Site, UK" Minerals 12, no. 7: 848. https://doi.org/10.3390/min12070848