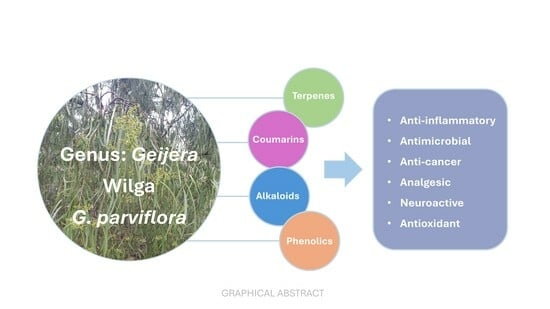
A Review of the Ethnobotanical Use, Chemistry and Pharmacological Activities of Constituents Derived from the Plant Genus Geijera (Rutaceae)
Abstract
:1. Introduction
2. Methodology
3. Chemical Constituents in Geijera Species
3.1. Coumarins
| Compound and Exact Mass (Da) | Source | Method of Identification | Reference | Pharmacological Activity of Compound (Various Sources) |
|---|---|---|---|---|
| 1 umbelliferone | G. salicifolia (leaves) | Melting point, IR and 1H NMR | [37] | Anti-inflammatory, antinociceptive, anti-hyperglycaemic, antibacterial, antifungal, inhibition of DPPH, hydroxyl, superoxide anion and ABTS radicals, molluscicide, antifeedant, anti-tumour, antimutagenic, fluorescent (sunscreen agent), bone-protective, anti-biofilm [38,39,40] |
 | ||||
| 162.0317 | ||||
| 2 geiparvarin | G. parviflora (leaves) | Combustion analysis, chemical derivatisation, UV, IR (G.p) | [20,37] | Anti-cancer, monoamine oxidase B inhibitor [41,42,43] |
 | ||||
| 326.1154 | G. salicifolia (leaves) | IR and 1H NMR (G.s) | ||
| 3 auraptene | G. parviflora (fruit/seeds) | IR and 1H NMR | [44] | Increases collagen I expression, anti-bacterial, anti-fungal, antileishmanial, anti-cancer, and antioxidant [45,46] |
 | ||||
| 298.1569 | ||||
| 4 marmin | G. parviflora (fruit/seeds) | IR and 1H NMR | [44] | No significant anti-inflammatory activity [47] |
 | ||||
| 332.1624 | ||||
| 5 6′-dehydromarmin | G. parviflora (fruit/seeds) | IR and 1H NMR | [44] | Anti-inflammatory, cytotoxic [10] |
 | ||||
| 330.1467 | ||||
| 6 2′,3′-dihydrogeiparvarin | G. parviflora (fruit/seeds) | IR and 1H NMR | [44,48] | Anti-cancer [48,49] |
 | G. salicifolia (leaves) | |||
| 330.1467 | ||||
| 7 (R)-6-O-(4-geranyloxy-2-hydroxy) cinnamoylmarmin | G. parviflora (leaves) | 2D NMR | [10] | Cytotoxic, anti-inflammatory [10] |
 | ||||
| 630.3193 | ||||
| 8 parvifloranine A | G. parviflora (leaves) | 2D NMR, ECD and MS | [50] | Anti-inflammatory [50] |
 | ||||
| 453.1788 | ||||
| 9 parvifloranine B | G. parviflora (leaves) | 2D NMR, ECD and MS | [50] | No significant anti-inflammatory activity [50] |
 | ||||
| 470.1689 | ||||
| 10 geijerin | G. salicifolia (bark) | Chemical derivatisation, UV, and IR | [37,51] | Acetylcholinesterase inhibitor [52] |
 | G. parviflora (leaves) | Melting point, IR and 1H NMR | ||
| 260.1049 | ||||
| 11 scoparone | G. parviflora (leaves) | GC-MS | [21] | Antifungal, anti-inflammatory, antioxidant, anti-apoptotic, anti-fibrotic, and hypolipidemic [53,54] |
 | ||||
| 206.0579 | ||||
| 12 suberosin | G. parviflora (leaves) | GC-MS | [21] | Anti-inflammatory and anticoagulant [55,56] |
 | ||||
| 244.1099 | ||||
| 13 dehydrogeijerin | G. parviflora (leaves) | Chemical derivatisation, UV, and IR (G.p) | [20,37] | Anti-inflammatory activity, acetylcholinesterase inhibitor [52,57] |
 | G. salicifolia (leaves) | IR and 1H NMR (G.s) | ||
| 258.0892 | ||||
| 14 6-(methoxyl) geiparvarin | G. parviflora (leaves) | 13C and 1H NMR | [10] | Anti-inflammatory, cytotoxic [10] |
 | ||||
| 356.1260 | ||||
| 15 osthole | G. parviflora (leaves) | GC-MS | [22] | Antitumour, anti-inflammatory, neuroprotective, anxiolytic, osteogenic, cardiovascular protective, antimicrobial, antiparasitic [58,59] |
 | ||||
| 244.1099 | ||||
| 16 angelicin (isopsoralen) | G. parviflora (leaves) | GC-MS | [22] | Anti-cancer, pro-osteogenic, antiviral, pro-chondrogenic, anti-inflammatory, erythroid differentiating, anti-periodontitis [60,61] |
 | ||||
| 186.0317 | ||||
| 17 xanthyletine | G. parviflora (leaves) | GC-MS | [22] | Antimicrobial, fungicide [62,63] |
 | ||||
| 228.0786 | ||||
| 18 luvangetin | G. balansae (leaves) | UV, IR, 1H NMR, MS | [28] | Antiulcer, antifungal, anti-inflammatory, antibacterial [64,65] |
 | ||||
| 258.0892 | ||||
| 19 xanthoxyletin | G. balansae (bark) | UV, IR, 1H NMR, MS | [28] | Anticonvulsant, anti-inflammatory, carbonic anhydrase inhibitor, anti-malaria, histone lysine methyltransferase G9a inhibitor [66,67] |
 | ||||
| 258.0892 | ||||
3.2. Alkaloids
| Compound and Exact Mass (Da) | Source | Method of Identification | Reference | Pharmacological Activity of Compound (Various Sources) |
|---|---|---|---|---|
| 20 11′-hexadecenoyl anthranilic acid | G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | [68] | Antibacterial vs. Gram positive bacteria [68] |
 | ||||
| 373.2617 | ||||
| 21 9′-hexadecenoyl anthranilic acid | G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | [68] | Antibacterial vs. Gram positive bacteria [68] |
 | ||||
| 373.2617 | ||||
| 22 7′-hexadecenoyl anthranilic acid | G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | [68] | Antibacterial vs. Gram positive bacteria [68] |
 | ||||
| 373.2617 | ||||
| 23 9,12,15-octadecatrienoyl anthranilic acid | G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | [68] | Did not display significant antibacterial activity [68] |
 | ||||
| 383.2460 | ||||
| 24 hexadecanoyl anthranilic acid | G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | [68] | Antibacterial vs. Gram positive bacteria [68] |
 | ||||
| 375.2773 | ||||
| 25 dictamnine | G. balansae (wood/bark) | 1H NMR, IR, UV, and MS | [28] | Antibacterial, antiviral, antifungal, antiprotozoal, anti-cancer, anti-inflammatory, antioxidant, cardiovascular, antiplatelet, antiosteoporosis, anti-anaphylactoid [72] |
 | ||||
| 199.0633 | ||||
| 26 skimmianine | G. salicifolia (leaves) | IR, melting point (G.s) | [28,69] | Anti-inflammatory, acetylcholinesterase inhibitor, anti-cancer [73,74,75] |
 | G. balansae (wood/bark) | 1H NMR, IR, UV, and MS (G.b) | ||
| 259.0845 | ||||
| 27 γ-fagarine | G. salicifolia (leaves) | IR, melting point (G.s) | [28,69] | Antileishmanial [76] |
 | G. balansae (wood/bark) | 1H NMR, IR, UV, and MS (G.b) | ||
| 229.0739 | ||||
| 28 platydesmine | G. salicifolia (leaves) | Melting point, combustion analysis, chemical degradation, IR, UV and 1H NMR (G.s) | [28,69] | Antifungal [77] |
 | ||||
| 259.1208 | G. balansae (leaves) | 1H NMR, IR, UV, and MS (G.b) | ||
| 29 platydesmine acetate | G. salicifolia (leaves) | Combustion analysis, chemical degradation, IR and 1H NMR | [69] | No activity reported to date |
 | ||||
| 301.1314 | ||||
| 30 flindersine | G. parviflora (fruit/seeds) | IR and melting point (G.p) | [28,44] | Anti-inflammatory, collagen III suppression, antibacterial, antifungal [10,45,78] |
 | G. balansae (leaves) | 1H NMR, IR, UV, and MS (G.b) | ||
| 227.0946 | ||||
| 31 4′-hydroxy-3′,4′-dihydroflindersine | G. balansae (leaves) | Chemical synthesis/derivatisation, 1H NMR, IR, UV, and MS | [28] | No activity reported to date |
 | ||||
| 245.1052 | ||||
| 32 cis-3′,4′-dihydroxy-3′,4′-dihydroflindersine | G. balansae (leaves) | Chemical synthesis/derivatisation, 1H NMR, IR, UV, and MS | [28] | No activity reported to date |
 | ||||
| 261.1001 | ||||
| 33 zanthobungeanine | G. balansae (leaves) | 1H NMR, IR, UV, and MS | [28] | Leishmanicidal activity on Leishmania Viannia panamensis intracellular amastigotes (EC50: 8.7 µg/)mL and promastigotes (EC50: 14.3 µg/)mL, respectively [79] |
 | ||||
| 271.1208 | ||||
| 34 8-(methoxyl)-flindersine | G. parviflora (leaves) | UV, IR, 2D NMR and MS | [10] | No activity reported to date |
 | ||||
| 257.1052 | ||||
| 35 N-(acetoxymethyl) flindersine | G. parviflora (leaves) | UV, IR, 2D NMR and MS | [10] | Anti-inflammatory, collagen III suppression [10,45] |
 | ||||
| 299.1158 | ||||
| 36 haplaphine | G. parviflora (leaves) | UV, IR, 2D NMR and MS (G.p) | [10,28] | Anti-inflammatory, cytotoxic [10] |
 | G. balansae (bark) | 1H NMR, IR, UV, and MS (G.b) | ||
| 229.1103 | ||||
| 37 4-methoxy N-methyl-2-quinolone | G. balansae (bark) | 1H NMR, IR, UV, and MS | [28] | Antimicrobial against MRSA, IC50 8.0 µM [80] |
 | ||||
| 189.0790 | ||||
| 38 geibalansine | G. balansae (leaves) | Chemical synthesis/derivatisation, 1H NMR, IR, UV, and MS | [27] | Antispasmodic [81] |
 | ||||
| 259.1208 | ||||
| 39 O-acetyl geibalansine | G. balansae (leaves) | Chemical derivatisation, 1H NMR, IR, UV, and MS | [27] | No activity reported to date |
 | ||||
| 301.1314 | ||||
| 40 geijedimerine | G. balansae (leaves) | Chemical derivatisation, 1H NMR, IR, UV, and MS | [28] | No activity reported to date |
 | ||||
| 454.1893 | ||||
| 41 hordenine | G. balansae (leaves) | 1H NMR, IR, UV, and MS | [27] | Diuretic, disinfectant, antihypotensive agent. Used for treatment of dysentery. Antifeedant for grasshoppers [67]. |
 | ||||
| 165.1154 |
3.3. Terpenes and Terpenoids
| Compound and Exact Mass (Da) | Source | Method of Identification | Reference | Pharmacological Activity of Compound (Various Sources) |
|---|---|---|---|---|
| 42 (E)-β-ocimene | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Anticonvulsant, antifungal, antitumour, plant pest resistance and attraction of plant pollinators (semiochemical) [83] |
 | ||||
| 136.1252 | ||||
| 43 (Z)-β-ocimene | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Anticonvulsant, antifungal, antitumour, plant pest resistance and attraction of plant pollinators (semiochemical) [83] |
 | ||||
| 136.1252 | ||||
| 44 myrcene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Sedative, muscle relaxant, anti-inflammatory, analgesic, anti-tumour, antioxidant, psychotropic, antibiotic, antimutagenic [84,85] |
 | ||||
| 136.1252 | ||||
| 45 limonene | G. salicifolia (leaves) | GC-MS | [16] | Anxiolytic, anti-carcinogenic [84] |
 | ||||
| 136.1252 | ||||
| 46 α-terpinene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Antioxidant, antimicrobial, acetylcholinesterase inhibition, sedative [85,86] |
 | ||||
| 136.1252 | ||||
| 47 γ-terpinene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Antioxidant, antimicrobial, acetylcholinesterase inhibition, antinociceptive, anti-inflammatory [86,87,88] |
 | ||||
| 136.1252 | ||||
| 48 terpinolene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Antioxidant, antimicrobial, larvicide, insecticide [86,89] |
 | ||||
| 136.1252 | ||||
| 49 α-pinene | G. parviflora (leaves) G. salicifolia (leaves) | Chemical derivatisation (G.p) GC-MS (G.s) | [16,18] | Anti-inflammatory, anti-tumour [84] |
 | ||||
| 136.1252 | ||||
| 50 β-pinene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Anti-inflammatory, anti-tumour [84] |
 | ||||
| 136.1252 | ||||
| 51 camphene | G. parviflora (leaves) | Chemical derivatisation | [18] | Antioxidant [90] |
 | ||||
| 136.1252 | ||||
| 52 sabinene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Antioxidant, anti-inflammatory [91,92] |
 | ||||
| 136.1252 | ||||
| 53 α-phellandrene | G. parviflora (leaves) | GC-MS | [16] | Antinociceptive, hyperthermic, promotes immune response, anti-cancer, antimicrobial, fungicide, pesticide [93] |
 | ||||
| 136.1252 | ||||
| 54 β-phellandrene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Acetylcholinesterase inhibitor, antifungal, expectorant [94,95] |
 | ||||
| 136.1252 | ||||
| 55 p-cymene | G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Antioxidant, anti-inflammatory, anti-cancer, antimicrobial [96] |
 | ||||
| 134.1096 | ||||
| 56 citronellyl acetate | G. linearifolia (leaves) | GC-MS | [16] | Pro-apoptotic activity in HepG2, fungicide, larvicide, bactericide, insect repellent/insecticide, antinociceptive [97] |
 | ||||
| 196.1620 | ||||
| 57 geranyl acetate | G. linearifolia (leaves) | GC-MS | [16] | Anti-cancer, antifungal [98,99] |
 | ||||
| 196.1463 | ||||
| 58 neryl acetate | G. linearifolia (leaves) | GC-MS | [16] | Fragrance and flavouring agent, strengthens skin barrier function [67,100] |
 | ||||
| 196.1463 | ||||
| 59 nerol | G. linearifolia (leaves) | GC-MS | [16] | Antimicrobial [101] |
 | ||||
| 154.1358 | ||||
| 60 geraniol | G. linearifolia (leaves) | GC-MS | [16] | Antimicrobial [101] |
 | ||||
| 154.1358 | ||||
| 61 linalool | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Anxiolytic, antibacterial, anti-inflammatory [102,103,104] |
 | ||||
| 154.1358 | ||||
| 62 α-terpineol | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Antioxidant, anti-cancer, anticonvulsant, antiulcer, antihypertensive, antinociceptive, enhances skin penetration, insecticidal properties [105] |
 | ||||
| 154.1358 | ||||
| 63 terpinen-4-ol | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Anti-inflammatory, antifungal, anti-cancer, antibacterial [106,107,108,109,110] |
 | ||||
| 154.1358 | ||||
| 64 1,8-cineole (eucalyptol) | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Anti-inflammatory, antioxidant, analgesic, antifungal [107,111,112] |
 | ||||
| 154.1358 | ||||
| 65 camphor | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [22] | Insecticidal, antimicrobial, antiviral, anticoccidial, antinociceptive, anti-cancer, antitussive, skin penetration enhancer [113] |
 | ||||
| 152.1201 | ||||
| 66 borneol | G. salicifolia (leaves) | GC-MS | [22] | Enhances membrane permeability, antibacterial, antifungal, antispasmodic, choleretic, acesodyne, sedative [114,115] |
 | ||||
| 154.1358 | ||||
| 67 azulene | G. parviflora (leaves) | Chemical derivatisation | [18] | Anti-inflammatory [116] |
 | ||||
| 128.0626 | ||||
| 68 pregeijerene | G. salicifolia (leaves) G. parviflora (leaves) | Chemical derivatisation, degradative analysis, and UV | [29] | Antifeedant, oviposition deterrence [117] |
 | ||||
| 162.1409 | ||||
| 69 cogeijerene | G. salicifolia (leaves) | Chemical derivatisation, degradative analysis, and UV (G.s) | [29,118] | No activity reported to date |
 | ||||
| 162.1409 | G. parviflora (leaves) | Chemical derivatisation, degradative analysis, IR, and UV (G.p) | ||
| 70 geijerene | G. parviflora (leaves) | Combustion analysis, chemical derivatisation, degradative analysis, IR (G.p) | [16,18,119] | Antifeedant, oviposition deterrence [117] |
 | ||||
| 164.1565 | G. salicifolia (leaves) | GC-MS (G.s) | ||
| 71 viridiflorene (ledene) | G. linearifolia (leaves) | GC-MS | [16] | Antifungal [120] |
 | ||||
| 204.1878 | ||||
| 72 α-selinene | G. parviflora (leaves) | GC-MS | [16] | No activity reported to date |
 | ||||
| 204.1878 | ||||
| 73 β-selinene | G. parviflora (leaves) | GC-MS | [16] | No activity reported to date |
 | ||||
| 204.1878 | ||||
| 74 selina-3, 7(11)-diene | G. parviflora (leaves) | GC-MS | [22] | No activity reported to date |
 | ||||
| 204.1878 | ||||
| 75 germacrene B | G. salicifolia (leaves) | GC-MS | [22] | Antimicrobial activity against Gram negative bacteria [121] |
 | ||||
| 204.1878 | ||||
| 76 germacrene D | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16,22] | Anti proliferative, scavenging activity towards the ABTS radical, antibacterial, antifungal, insecticidal, repels herbivores, attracts pollinators [122,123] |
 | ||||
| 208.2191 | ||||
| 77 bicyclogermacrene | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Larvicidal activity [124] |
 | ||||
| 204.1878 | ||||
| 78 α-bergamotene | G. parviflora (leaves) | GC-MS | [16] | Antifeedant [125] |
 | ||||
| 204.1878 | ||||
| 79 δ-cadinene | G. parviflora (leaves) | GC-MS | [16] | Acaricidal, antiproliferative and apoptotic [126,127] |
 | ||||
| 204.1878 | ||||
| 80 β-elemene | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Anti-cancer, antineoplastic, reproductive toxicity [128,129] |
 | ||||
| 204.1878 | ||||
| 81 γ-elemene | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Larvicidal activity [130] |
 | ||||
| 204.1878 | ||||
| 82 α-caryophyllene (humulene) | G. salicifolia (leaves) | GC-MS | [22] | Antibacterial, anti-inflammatory, antitumor, analgesic [131,132,133] |
 | ||||
| 204.1878 | ||||
| 83 β-caryophyllene | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Anti-inflammatory, analgesic, antimalarial, antifungal, antibacterial, anti-tumour [84,134] |
 | ||||
| 204.1878 | ||||
| 84 α-santalene | G. parviflora (leaves) | GC-MS | [22] | Insect repellent, semiochemical [125] |
 | ||||
| 204.1878 | ||||
| 85 aromadendrene | G. parviflora (leaves) G. linearifolia (leaves) G. salicifolia (leaves) | GC-MS | [16,22] | Antibacterial (MRSA and drug resistant pathogens) [135] |
 | ||||
| 204.1878 | ||||
| 86 (E,E)-α-farnesene | G. parviflora (leaves) G. linearifolia (leaves) G. salicifolia (leaves) | GC-MS | [16] | Semiochemical, antibacterial, anticariogenic, anti-cancer, anti-plasmodial, hepatoprotective, antioxidant, anti-inflammatory, antifungal [136,137] |
 | ||||
| 204.1878 | ||||
| 87 (E,E)-farnesol | G. linearifolia (leaves) | GC-MS | [16] | Antibacterial, antifungal [138,139] |
 | ||||
| 222.1984 | ||||
| 88 guaiol | G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [22] | Insecticide, antimicrobial, acaricidal, anti-cancer [140,141,142] |
 | ||||
| 208.1827 | ||||
| 89 elemol | G. parviflora (leaves) | GC-MS | [16] | Antifungal [143] |
 | G. salicifolia (leaves) | |||
| 222.1984 | ||||
| 90 palustrol | G. linearifolia (leaves) | GC-MS | [16] | Semiochemical [144] |
 | ||||
| 222.1984 | ||||
| 91 ledol | G. parviflora (leaves) | GC-MS | [22] | Antifungal, toxic CNS effects, antitussive, expectorant [145,146] |
 | ||||
| 222.1984 | ||||
| 92 globulol | G. parviflora (leaves) | GC-MS | [16] | Antimicrobial [147] |
 | ||||
| 222.1984 | ||||
| 93 epi-globulol | G. parviflora (leaves) | GC-MS | [16] | Antimicrobial, semiochemical [148] |
 | ||||
| 222.1984 | ||||
| 94 τ-cadinol | G. linearifolia (leaves) | GC-MS | [16] | Antitrypanosomal, smooth muscle relaxant, inhibits effects of cholera toxins [149,150] |
 | ||||
| 207.1749 | ||||
| 95 α-eudesmol | G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Antitrypanosomal, anti-cancer, anti-neurogenic inflammation [151,152,153] |
 | ||||
| 222.1984 | ||||
| 96 β-eudesmol | G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Anti-cancer, sedative, hepatoprotective, anti-inflammatory, diuretic, inhibits platelet aggregation, insect repellent, anti-allergy [67,152,154,155,156,157] |
 | ||||
| 222.1984 | ||||
| 97 γ-eudesmol | G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16] | Anti-cancer [152] |
 | ||||
| 222.1984 | ||||
| 98 viridiflorol | G. parviflora (leaves) | GC-MS | [16] | Anti-mycobacterial, anti-inflammatory, antioxidant [158] |
 | ||||
| 222.1984 | ||||
| 99 (E,E)-farnesal | G. linearifolia (leaves) | GC-MS | [16] | Semiochemical [159] |
 | ||||
| 220.1827 | ||||
| 100 caryophyllene oxide | G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) | GC-MS | [16] | Anti-cancer, analgesic [134] |
 | ||||
| 220.1827 | ||||
| 101 caryophylla-4(12), 8(13)-dien-5-ol | G. parviflora (leaves) | GC-MS | [22] | No activity reported to date |
 | ||||
| 220.1827 | ||||
| 102 spathulenol | G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) | GC-MS | [16,22] | Antioxidant, anti-inflammatory, antiproliferative, antimycobacterial, antimicrobial [160,161] |
 | ||||
| 220.1827 | ||||
| 103 eremophilone | G. parviflora (leaves) | GC-MS | [16,22] | Cytotoxic, insecticidal, insect repellent, antifeedant (against termites) [162,163] |
 | ||||
| 218.1671 | ||||
| 104 cyclocolorenone | G. parviflora (leaves) | GC-MS | [16,22] | Antifeedant, antimicrobial, allelopathic, anti-inflammatory, insect repellent [164] |
 | ||||
| 218.1671 | ||||
| 105 β-sitosterol | G. salicifolia (leaves) | Melting point and IR | [37] | Anti-cancer, anthelminthic, antimutagenic [165,166] |
 | ||||
| 414.3862 |
3.4. Miscellaneous Compounds Isolated
| Compound and Exact Mass (Da) | Source | Method of Identification | Reference | Pharmacological Activity of Compound (Various Sources) |
|---|---|---|---|---|
| 106 brevifolin (xanthoxylin) | G. parviflora (leaves) G. balansae (bark) G. salicifolia (leaves) | GC-MS (G.p) 1H NMR, IR, UV, and MS (G.b) Melting point (G.s) | [16,18,28] | Antioxidant, hepatoprotective, antibacterial, antifungal, antinociceptive, antiedematogenic and antispasmodic [167,168] |
 | ||||
| 196.0736 | ||||
| 107 elemicin | G. parviflora (leaves) | GC-MS | [9] | Psychotropic, antimicrobial, antioxidant, acetylcholinesterase inhibitor, antiviral [9,169,170] |
 | ||||
| 208.1099 | ||||
| 108 3,5,8,4′-tetrahydroxy-6,7-dimethoxyflavone | G. parviflora (leaves) | 1H and 13C NMR | [10] | No activity reported to date |
 | ||||
| 346.0689 | ||||
| 109 2-phenylethyl isobutyrate | G. parviflora (leaves) | 1H and 13C NMR | [10] | Odorant [171] |
 | ||||
| 192.1150 | ||||
| 110 isoamyl isovalerate | G. parviflora (leaves) | 1H and 13C NMR | [10] | Flavouring/odorant [172] |
 | ||||
| 172.1463 | ||||
| 111 cis-jasmone | G. parviflora (leaves) | GC-MS | [22] | Semiochemical [173] |
 | ||||
| 164.1201 | ||||
| 112 methyl eugenol | G. parviflora (leaves) | GC-MS | [22] | Attracts pollinator insects (semiochemical) [174] |
 | ||||
| 178.0994 | ||||
| 113 phthalic acid | G. parviflora (leaves) | GC-MS | [22] | Endocrine disruptor [175] |
 | ||||
| 166.0266 | ||||
| 114 vanillin | G. balansae (wood) | 1H NMR, IR, UV, and MS | [28] | Flavouring, pharmaceutical excipient, antioxidant, inhibits lipid peroxidation [67] |
 | ||||
| 152.0473 | ||||
| 115 methyl syringate | G. balansae (wood) | 1H NMR, IR, UV, and MS | [28] | Anti-diabetic, TRPA1 agonist [176,177] |
 | ||||
| 212.0685 | ||||
| 116 methyl ferulate | G. balansae (wood) | 1H NMR, IR, UV, and MS | [28] | Inhibits COX-2 expression, blocks p-p38 and p-JNK in primary bone marrow derived-macrophages [178,179] |
 | ||||
| 208.0736 | ||||
| 117 ethyl ferulate | G. balansae (wood) | 1H NMR, IR, UV, and MS | [28] | Antioxidative, antiapoptotic, antirheumatic, neuroprotective and anti-inflammatory [180,181] |
 | ||||
| 222.0892 |
4. Pharmacological Activities of Geijera Constituents
|
|
4.1. Geijera Secondary Metabolites That Can Be Linked to Its Ethnobotanical Uses
- anti-inflammatory activity
- analgesic/antinociceptive activity
- antimicrobial, antifungal, and antioxidant activity
- acetylcholinesterase inhibition, monoamine oxidase inhibition, muscle relaxant activity, sedative activity, anticonvulsant activity, and psychotropic activity (from neuro- and psycho-active compounds).
4.1.1. Anti-Inflammatory, Analgesic, and Antinociceptive Compounds
4.1.2. Antimicrobial, Antifungal, and Antioxidant Compounds
4.1.3. Neuroactive and Psychoactive Compounds
4.1.4. Anti-Cancer Compounds
4.1.5. Compounds That Offer Pest Resistance, Insecticidal and Semiochemical Benefits
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPNI. International Plant Names Index. Available online: https://www.ipni.org/?f=&sort=published_desc&q=Geijera (accessed on 12 December 2023).
- POWO. Geijera Schott|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:35743-1 (accessed on 12 December 2023).
- ALA. Genus: Geijera. Available online: https://bie.ala.org.au/species/https://id.biodiversity.org.au/node/apni/2905474 (accessed on 12 December 2023).
- Hartley, T.G. Flora of Australia: Meliaceae, Rutaceae, Zygophyllaceae; ABRS/CSIRO Australia: Clayton, VIC, Australia, 2013; Volume 26.
- Bruy, D.; Lannuzel, G.; Gâteblé, G.; Munzinger, J. Three new species threatened by mining activity in New Caledonia. Phytotaxa 2023, 578, 228–240. [Google Scholar] [CrossRef]
- Kubitzki, K.; Kallunki, J.A.; Duretto, M.; Wilson, P.G. Rutaceae; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 276–356. [Google Scholar]
- Packer, J.; Turpin, G.; Ens, E.; Venkataya, B.; Hunter, J.; Mbabaram, C.; Yirralka, R. Building partnerships for linking biomedical science with traditional knowledge of customary medicines: A case study with two Australian Indigenous communities. J. Ethnobiol. Ethnomed. 2019, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.L. The Euahlayi Tribe: A Study of Aboriginal Life in Australia; Project Gutenberg: Salt Lake City, UT, USA, 1905. [Google Scholar]
- Sadgrove, N.J.; Jones, G.L. Characterization and Bioactivity of Essential Oils from Geijera parviflora (Rutaceae): A Native Bush Medicine from Australia. Nat. Prod. Commun. 2013, 8, 747–751. [Google Scholar] [CrossRef]
- Banbury, L.K.; Shou, Q.; Renshaw, D.E.; Lambley, E.H.; Griesser, H.J.; Mon, H.; Wohlmuth, H. Compounds from Geijera parviflora with prostaglandin E2 inhibitory activity may explain its traditional use for pain relief. J. Ethnopharmacol. 2015, 163, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Cribb, A.B.; Cribb, J.W. Wild Medicine in Australia; Collins: Sydney, NSW, Australia, 1981. [Google Scholar]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; New Holland: Sydney, NSW, Australia, 2001. [Google Scholar]
- Ecological Cultural Knowledge-Paakantyi (Barkindji) Knowledge Shared by the Paakantyi (Barkindji) People. Available online: https://www.lls.nsw.gov.au/__data/assets/pdf_file/0007/1419784/WLLS-Paakantyi-Booklet-PURPLE-22.pdf (accessed on 12 December 2023).
- Ecological Cultural Knowledge-Ngiyampaa Knowledge Shared by the Ngiyampaa People. Available online: https://www.lls.nsw.gov.au/__data/assets/pdf_file/0003/737625/Ngiyampaa_Booklet_WEB-updated.pdf (accessed on 12 December 2023).
- Williams, A.; Sides, T. Wiradjuri Plant Use in the Murrumbidgee Catchment. Available online: https://www.lls.nsw.gov.au/__data/assets/pdf_file/0009/1270845/Wiradjuri-Plant-Use-in-the-Murrumbidgee-Catchment.pdf (accessed on 14 December 2023).
- Brophy, J.J.; Goldsack, R.J. The Leaf Oils of Coatesia and Geijera (Rutaceae) from Australia. J. Essent. Oil Res. 2005, 17, 169–174. [Google Scholar] [CrossRef]
- Penfold, A.R. Natural Chemical Resources of Australian Plant Products. Part II. J. Chem. Educ. 1932, 9, 429–438. [Google Scholar] [CrossRef]
- Penfold, A.R.; Simonsen, J.L. The essential oils of three species of Geijera and the occurrence of a new hydrocarbon. Part I. J. Proc. R. Soc. N. S. W. 1930, 63–64, 264–297. [Google Scholar] [CrossRef]
- Penfold, A.R.; Simonsen, J.L. The essential oils of three species of Geijera and the occurrence of a new hydrocarbon, Part II. J. Proc. R. Soc. N. S. W. 1932, 65–66, 332. [Google Scholar] [CrossRef]
- Lahey, F.N.; MacLeod, J.K. The Coumarins of Geijera Parviflora Lindl. Aust. J. Chem. 1967, 20, 1943–1955. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Lyddiard, D.; Jones, G.L. Bioactive volatiles from Geijera parviflora Lindl. (Rutaceae): Evidence for coumarin chemotypes. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): V World 1125, Brisbane, Australia, 25 October 2016; pp. 145–150. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Goncalves-Martins, M.; Jones, G.L. Chemogeography and antimicrobial activity of essential oils from Geijera parviflora and Geijera salicifolia (Rutaceae): Two traditional Australian medicinal plants. Phytochemistry 2014, 104, 60–71. [Google Scholar] [CrossRef]
- Floyd, A.G. Research Note No. 30 N.S.W. Rainforest Trees Part IV Family Rutaceae; Forestry Commission of New South Wales: Sydney, NSW, Australia, 1979; p. 72. [Google Scholar]
- Bodkin, F. Dharawal Pharmacopeia Collection; Western Sydney University: Penrith, NSW, Australia, 2021. [Google Scholar]
- Appelhans, M.S.; Bayly, M.J.; Heslewood, M.M.; Groppo, M.; Verboom, G.A.; Forster, P.I.; Kallunki, J.A.; Duretto, M.F. A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. Taxon 2021, 70, 1035–1061. [Google Scholar] [CrossRef]
- Waterman, P.G. Alkaloids of the rutaceae: Their distribution and systematic significance. Biochem. Syst. Ecol. 1975, 3, 149–180. [Google Scholar] [CrossRef]
- Ahond, A.; Poupat, C.; Pusset, J. Geibalansine et O-acétylgeibalansine, nouveaux alcaloïdes isolés de Geijera balansae. Phytochemistry 1979, 18, 1415–1416. [Google Scholar] [CrossRef]
- Mitaku, S.; Skaltsounis, A.-L.; Tillequin, F.; Koch, M.; Pusset, J.; Chauviere, G. Plantes de Nouvelle-Calédonie, XCVI. Alcaloïdes de Geijera balansae. J. Nat. Prod. 1985, 48, 772–777. [Google Scholar] [CrossRef]
- Jones, R.V.J.; Sutherland, M.D. Terpenoid chemistry. XV. 1,5-Dimethylcyclodeca-1,5,7-triene, the precursor of geijerene in Geijera parviflora (Lindley). Aust. J. Chem. 1968, 21, 2255–2264. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Xu, W.; Farzaneh, F.; Xu, R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.I.; Waterman, P.G. Coumarins in the Rutaceae. Phytochemistry 1978, 17, 845–864. [Google Scholar] [CrossRef]
- Levin, D.A. The Chemical Defenses of Plants to Pathogens and Herbivores. Annu. Rev. Ecol. Syst. 1976, 7, 121–159. [Google Scholar] [CrossRef]
- Yamane, H.; Konno, K.; Sabelis, M.; Takabayashi, J.; Sassa, T.; Oikawa, H. Chemical Defence and Toxins of Plants; Elsevier: Amsterdam, The Netherlands, 2010; pp. 339–385. [Google Scholar]
- Kingsbury, J.M. The Evolutionary and Ecological Significance of Plant Toxins. In Handbook of Natural Toxins Plant and Fungal Toxins; Keeler, R.F., Tu, A.T., Eds.; Marcel Dekker: New York, NY, USA, 1983; Volume 1. [Google Scholar]
- Shimizu, B.-I. 2-Oxoglutarate-dependent dioxygenases in the biosynthesis of simple coumarins. Front. Plant Sci. 2014, 5, 549. [Google Scholar] [CrossRef]
- Valenti, P.; Rampa, A.; Recanatini, M.; Bisi, A.; Belluti, F.; Da Re, P.; Carrara, M.; Cima, L. Synthesis, cytotoxicity and SAR of simple geiparvarin analogues. Anticancer Drug Des. 1997, 12, 443–451. [Google Scholar]
- Ritchie, E.; Taylor, W.C.; Young, L.M. Some extractives from Geijera salicifolia Schott. Aust. J. Chem. 1968, 21, 1381. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. Chapter Eleven—Medicinal natural products in osteoporosis. In Annual Reports in Medicinal Chemistry; Sarker, S.D., Nahar, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 55, pp. 327–372. [Google Scholar]
- Rauf, A.; Khan, R.; Khan, H.; Pervez, S.; Pirzada, A.S. In vivo antinociceptive and anti-inflammatory activities of umbelliferone isolated from Potentilla evestita. Nat. Prod. Res. 2014, 28, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Chimichi, S.; Boccalini, M.; Salvador, A.; Dall’Acqua, F.; Basso, G.; Viola, G. Synthesis and biological evaluation of new geiparvarin derivatives. ChemMedChem 2009, 4, 769–779. [Google Scholar] [CrossRef]
- Carotti, A.; Carrieri, A.; Chimichi, S.; Boccalini, M.; Cosimelli, B.; Gnerre, C.; Carotti, A.; Carrupt, P.A. Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies. Bioorg. Med. Chem. Lett. 2002, 12, 3551–3555. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Lee, A. Extractives of Geijera Parviflora. Phytochemistry 1972, 11, 763–767. [Google Scholar] [CrossRef]
- Adams, D.H.; Shou, Q.; Wohlmuth, H.; Cowin, A.J. Native Australian plant extracts differentially induce Collagen I and Collagen III in vitro and could be important targets for the development of new wound healing therapies. Fitoterapia 2016, 109, 45–51. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Tayarani-Najaran, N.; Eghbali, S. A Review of Auraptene as an Anticancer Agent. Front. Pharmacol. 2021, 12, 698352. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Liao, Y.-R.; Hung, H.-Y.; Chuang, C.-W.; Hwang, T.-L.; Huang, S.-C.; Shiao, Y.-J.; Kuo, D.-H.; Wu, T.-S. Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules 2017, 22, 967. [Google Scholar] [CrossRef]
- Padmawinata, K. Isolation and identification of cancer delaying compounds from the leaves of Geijera salicifolia. Acta Pharm. 1973, 4, 1–9. [Google Scholar]
- Davis, R.A.; Vullo, D.; Maresca, A.; Supuran, C.T.; Poulsen, S.A. Natural product coumarins that inhibit human carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Shou, Q.; Banbury, L.K.; Renshaw, D.E.; Smith, J.E.; He, X.; Dowell, A.; Griesser, H.J.; Heinrich, M.; Wohlmuth, H. Parvifloranines A and B, two 11-carbon alkaloids from Geijera parviflora. J. Nat. Prod. 2013, 76, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Lahey, F.N.; Wluka, D.J. Geijerin: A new coumarin from the bark of Geijera salicifolia Schott. Aust. J. Chem. 1955, 8, 125. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.P.; Kim, M.J.; Chun, W. Acetylcholinesterase inhibitors from Angelica polymorpha stem. Nat. Prod. Sci. 2017, 23, 97–102. [Google Scholar] [CrossRef]
- Ramírez-Pelayo, C.; Martínez-Quiñones, J.; Gil, J.; Durango, D. Coumarins from the peel of citrus grown in Colombia: Composition, elicitation and antifungal activity. Heliyon 2019, 5, e01937. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wang, X.; Yu, Z.; Fan, X.; Cui, B.; Zhao, T.; Mao, L.; Feng, H.; Lin, L.; Yu, Q.; et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol. Res. 2020, 160, 105170. [Google Scholar] [CrossRef]
- Golfakhrabadi, F.; Abdollahi, M.; Ardakani, M.R.; Saeidnia, S.; Akbarzadeh, T.; Ahmadabadi, A.N.; Ebrahimi, A.; Yousefbeyk, F.; Hassanzadeh, A.; Khanavi, M. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm. Biol. 2014, 52, 1335–1340. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tsai, W.J.; Wu, M.H.; Lin, L.C.; Kuo, Y.C. Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB. Br. J. Pharmacol. 2007, 150, 298–312. [Google Scholar] [CrossRef]
- Bae, D.S.; Kim, C.Y.; Lee, J.K. Anti-inflammatory effects of dehydrogeijerin in LPS-stimulated murine macrophages. Int. Immunopharmacol. 2012, 14, 734–739. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Xu, J.; Guan, S.; Sun, D. Research of the anxiolytic effect of osthol. Chin. Med. Her. 2012, 9, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, C.; Zhou, C.; Xu, D.; Qu, H.-b. Screening Antitumor Compounds Psoralen and Isopsoralen from Psoralea corylifolia L. Seeds. Evid.-Based Complement. Altern. Med. 2011, 2011, 363052. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Tan, L.T.H.; Lee, W.L.; Yap, W.H.; Pusparajah, P.; Low, L.E.; Tang, S.Y.; Chan, K.G.; Lee, L.H.; Goh, B.H. Angelicin—A Furocoumarin Compound with Vast Biological Potential. Front. Pharmacol. 2020, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kunesch, G.; Chuilon, S.; Ravisé, A. Structure and biological activity of xanthyletin: A new phytoalexin of CITRUS. Fruits 1985, 40, 807–811. [Google Scholar]
- Cazal Cde, M.; Domingues Vde, C.; Batalhão, J.R.; Bueno, O.C.; Filho, E.R.; da Silva, M.F.; Vieira, P.C.; Fernandes, J.B. Isolation of xanthyletin, an inhibitor of ants’ symbiotic fungus, by high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4307–4312. [Google Scholar] [CrossRef] [PubMed]
- Erst, A.S.; Chernonosov, A.A.; Petrova, N.V.; Kulikovskiy, M.S.; Maltseva, S.Y.; Wang, W.; Kostikova, V.A. Investigation of Chemical Constituents of Eranthis longistipitata (Ranunculaceae): Coumarins and Furochromones. Int. J. Mol. Sci. 2022, 23, 406. [Google Scholar] [CrossRef]
- Tuan Anh, H.L.; Kim, D.-C.; Ko, W.; Ha, T.M.; Nhiem, N.X.; Yen, P.H.; Tai, B.H.; Truong, L.H.; Long, V.N.; Gioi, T.; et al. Anti-inflammatory coumarins from Paramignya trimera. Pharm. Biol. 2017, 55, 1195–1201. [Google Scholar] [CrossRef]
- PubChem. Xanthoxyletin. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 14 December 2023).
- DNP. Dictionary of Natural Products. Available online: http://dnp.chemnetbase.com/dictionary-search.do?method=view&id=425027&si= (accessed on 15 December 2023).
- Shou, Q.; Banbury, L.K.; Maccarone, A.T.; Renshaw, D.E.; Mon, H.; Griesser, S.; Griesser, H.J.; Blanksby, S.J.; Smith, J.E.; Wohlmuth, H. Antibacterial anthranilic acid derivatives from Geijera parviflora. Fitoterapia 2014, 93, 62–66. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A. Alkaloids of Geijera salicifolia Schott. (family Rutaceae): The identification of platydesmine and platydesmine acetate. Aust. J. Chem. 1966, 19, 1991. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Szewczyk, A.; Pęczek, F. Furoquinoline Alkaloids: Insights into Chemistry, Occurrence, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 12811. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-D.; Zhang, D.-B.; Ren, J.; Yang, M.-J. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Med. Chem. Res. 2012, 21, 722–725. [Google Scholar] [CrossRef]
- Zuo, Y.; Pu, J.; Chen, G.; Shen, W.; Wang, B. Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Nat. Prod. Res. 2019, 33, 759–762. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Rojas de Arias, A.; Yaluff, G.; de Bilbao, N.V.; Nakayama, H.; Torres, S.; Schinini, A.; Guy, I.; Heinzen, H.; Fournet, A. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine 2010, 17, 375–378. [Google Scholar] [CrossRef]
- De Souza, R.C.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.d.G.; Godoy, M.F.; Pagnocca, F.C.; Bueno, O.C.; Hebling, M.J.A.; Pirani, J.R. A new imidazole alkaloid and other constituents from Pilocarpus grandiflorus and their antifungal activity. Z. Für Naturforschung B 2005, 60, 787–791. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J. Ethnopharmacol. 2009, 123, 494–498. [Google Scholar] [CrossRef]
- Coy Barrera, C.A.; Coy Barrera, E.D.; Granados Falla, D.S.; Delgado Murcia, G.; Cuca Suarez, L.E. Seco-limonoids and quinoline alkaloids from Raputia heptaphylla and their antileishmanial activity. Chem. Pharm. Bull. 2011, 59, 855–859. [Google Scholar] [CrossRef]
- Rodríguez-Guzmán, R.; Johansmann Fulks, L.C.; Radwan, M.M.; Burandt, C.L.; Ross, S.A. Chemical Constituents, Antimicrobial and Antimalarial Activities of Zanthoxylum monophyllum. Planta Med. 2011, 77, 1542–1544. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural Antispasmodics: Source, Stereochemical Configuration, and Biological Activity. Biomed. Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Chapter Three-Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 67–134. [Google Scholar]
- Louis, B.W.S. Cannabis as Medicine; Taylor & Francis Group: Milton, UK, 2019. [Google Scholar]
- Hazekamp, A.; Fischedick, J.T.; Díez, M.L.; Lubbe, A.; Ruhaak, R.L. 3.24-Chemistry of Cannabis. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 1033–1084. [Google Scholar]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Passos, F.F.d.B.; Lopes, E.M.; de Araújo, J.M.; de Sousa, D.P.; Veras, L.M.C.; Leite, J.R.S.A.; Almeida, F.R.d.C. Involvement of Cholinergic and Opioid System in γ-Terpinene-Mediated Antinociception. Evid.-Based Complement. Altern. Med. 2015, 2015, 829414. [Google Scholar] [CrossRef]
- Ramalho, T.; Pacheco de Oliveira, M.; Lima, A.; Bezerra-Santos, C.; Piuvezam, M. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254. [Google Scholar] [CrossRef]
- Menezes, I.O.; Scherf, J.R.; Martins, A.O.B.P.B.; Ramos, A.G.B.; Quintans, J.d.S.S.; Coutinho, H.D.M.; Ribeiro-Filho, J.; de Menezes, I.R.A. Biological properties of terpinolene evidenced by in silico, in vitro and in vivo studies: A systematic review. Phytomedicine 2021, 93, 153768. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants–Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Sci. Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Williams, C. Medicinal Plants in Australia Volume 2: Gums, Resins, Tannin and Essential Oils; Rosenberg Publishing: Kenthurst, NSW, Australia, 2011; p. 300. [Google Scholar]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.; Rocha, N.; Carvalho, A.; Vasconcelos, L.; Dias, M.; Sousa, D.; Sousa, F.; Fonteles, M. TRP and ASIC channels mediate the antinociceptive effect of citronellyl acetate. Chem.-Biol. Interact. 2013, 203, 573–579. [Google Scholar] [CrossRef]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G.J.J.B. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON 2018, 23, 346–352. [Google Scholar]
- Khayyat, S.A.; Sameeh, M.Y. Bioactive epoxides and hydroperoxides derived from naturally monoterpene geranyl acetate. Saudi Pharm. J. 2018, 26, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; Olivero, M.; Rouquet, V.; Moga, A.; Pagnon, A.; Cenizo, V.; Portes, P. Neryl acetate, the major component of Corsican Helichrysum italicum essential oil, mediates its biological activities on skin barrier. PLoS ONE 2023, 18, e0268384. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.S.; Denkova, Z.; Nikolova, R.; Geissler, M. Purity, Antimicrobial Activities and Olfactoric Evaluations of Geraniol/Nerol and Various of Their Derivatives. J. Essent. Oil Res. 2007, 19, 288–291. [Google Scholar] [CrossRef]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool Odor-Induced Anxiolytic Effects in Mice. Front. Behav. Neurosci. 2018, 12, 241. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 415–422. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chen, Y.-J.; Chen, J.J.W.; Shieh, J.-J.; Huang, C.-H.; Lin, P.-S.; Chang, G.-C.; Chang, J.-T.; Lin, C.-C. Terpinen-4-ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer in Vitro and in Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 818261. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Arancia, G.; Molinari, A.; Salvatore, G.; Mondello, F. Terpinen-4-ol, The Main Component of Melaleuca alternifolia (Tea Tree) Oil Inhibits the In Vitro Growth of Human Melanoma Cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256. [Google Scholar] [CrossRef]
- Yin, C.; Liu, B.; Wang, P.; Li, X.; Li, Y.; Zheng, X.; Tai, Y.; Wang, C.; Liu, B. Eucalyptol alleviates inflammation and pain responses in a mouse model of gout arthritis. Br. J. Pharmacol. 2020, 177, 2042–2057. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Qin, J.; Lu, W.; Wang, J. The Use of Borneol as an Enhancer for Targeting Aprotinin-Conjugated PEG-PLGA Nanoparticles to the Brain. Pharm. Res. 2013, 30, 2560–2572. [Google Scholar] [CrossRef]
- Tabanca, N.; Kırımer, N.; Demirci, B.; Demirci, F.; Başer, K.H.C. Composition and Antimicrobial Activity of the Essential Oils of Micromeria cristata subsp. phrygia and the Enantiomeric Distribution of Borneol. J. Agric. Food Chem. 2001, 49, 4300–4303. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Yuzer, A.; Ince, T.; Ince, M. Anti-Cancer and Anti-Inflammatory Activities of Bromo- and Cyano-Substituted Azulene Derivatives. Inflammation 2020, 43, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.R.; Reddy, A.S.; Devi, P.S.; Reddy, K.J. Insecticidal, antifeedant and oviposition deterrent effects of the essential oil and individual compounds from leaves of Chloroxylon swietenia DC. Pest Manag. Sci. 2006, 55, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.; Powell, V.; Sutherland, M.D. Constitution and biogenesis of two new sesquiterpenes. Tetrahedron Lett. 1961, 2, 763–767. [Google Scholar] [CrossRef]
- Sutherland, M.D. Terpenoid chemistry. VII. The structure of geijerene. Aust. J. Chem. 1964, 17, 75–91. [Google Scholar] [CrossRef]
- Mokhtari, M.; Jackson, M.D.; Brown, A.S.; Ackerley, D.F.; Ritson, N.J.; Keyzers, R.A.; Munkacsi, A.B. Bioactivity-Guided Metabolite Profiling of Feijoa (Acca sellowiana) Cultivars Identifies 4-Cyclopentene-1,3-dione as a Potent Antifungal Inhibitor of Chitin Synthesis. J. Agric. Food Chem. 2018, 66, 5531–5539. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini, D.; Maggi, F. Kundmannia sicula (L.) DC: A rich source of germacrene D. J. Essent. Oil Res. 2017, 29, 437–442. [Google Scholar] [CrossRef]
- Myron, K.; Clarkson, B.; Gemmill, C. New Zealand Journal of Botany Biological flora of New Zealand 16: Pittosporum kirkii Hook.f. ex Kirk, Kirk’s kōhūhū, thick-leaved kohukohu. N. Z. J. Bot. 2020, 59, 112–136. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2016, 133, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Park, Y.-L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef]
- Hui, L.-M.; Zhao, G.-D.; Zhao, J.-J. δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. Int. J. Clin. Exp. Pathol. 2015, 8, 6046. [Google Scholar] [PubMed]
- Guo, X.; Shang, X.; Li, B.; Zhou, X.Z.; Wen, H.; Zhang, J. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compund, δ-cadinene against Psoroptes cuniculi. Vet. Parasitol. 2017, 236, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Weng, S.; Lv, M.; Chen, W.; Bi, Z.; Chen, H.; Luo, T.; Hu, H.; Liao, W. β-Elemene Inhibits Human Sperm Function by Affecting Sperm Vitality and Intracellular Calcium. Cell. Physiol. Biochem. 2018, 51, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High toxicity of camphene and γ-elemene from Wedelia prostrata essential oil against larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. Int. 2018, 25, 10383–10391. [Google Scholar] [CrossRef]
- Pichette, A.; Larouche, P.L.; Lebrun, M.; Legault, J. Composition and antibacterial activity of Abies balsamea essential oil. Phytother. Res. 2006, 20, 371–373. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Chapter 53—Cannabis sativa and Hemp. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 735–754. [Google Scholar]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef]
- Ishnava, K.B.; Chauhan, J.B.; Barad, M.B. Anticariogenic and phytochemical evaluation of Eucalyptus globules Labill. Saudi J. Biol. Sci. 2013, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.D.; Mulange, S.V.; Kodgire, S.S.; Zore, G.B.; Karuppayil, S.M. Effects of Cinnamaldehyde, Ocimene, Camphene, Curcumin and Farnesene on Candida albicans. Adv. Microbiol. 2016, 06, 627–643. [Google Scholar] [CrossRef]
- Cugini, C.; Calfee, M.W.; Farrow, J.M.; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.M.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-Induced Apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.-J.; Xie, H.-Q.; Mu, Q. Guaiol—A Naturally Occurring Insecticidal Sesquiterpene. Nat. Prod. Commun. 2013, 8, 1934578X1300801001. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Senbill, H.; Van Wyk, B.-E.; Greatrex, B.W. New Labdanes with Antimicrobial and Acaricidal Activity: Terpenes of Callitris and Widdringtonia (Cupressaceae). Antibiotics 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, J.; Wu, J.; Huang, N.; Cui, Z.; Luo, Y.; Sun, F.; Pan, Q.; Li, Y.; Yang, Q. (−)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol. Ther. 2018, 19, 706–714. [Google Scholar] [CrossRef]
- Monteiro, A.F.M.; de Moura, É.P.; de Sousa, N.F.; Muratov, E.; Bezerra, A.H.R.; Scotti, M.T.; Scotti, L. Prediction of antifungal activity, cytotoxicity risks and molecular docking against Malassezia furfur of constituents of citronella essential oil (Cymbopogon winterianus). In Proceedings of the MOL2NET, International Conference Series on Multidisciplinary Sciences, UNC, Chape Hill, NC, USA, 20 March–20 December 2019. [Google Scholar]
- Jaenson, T.G.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of tick-repellent plants from Sweden. Med. Vet. Entomol. 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Jesionek, A.; Poblocka-Olech, L.; Zabiegala, B.; Bucinski, A.; Krauze-Baranowska, M.; Luczkiewicz, M. Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. J. Chromatogr. B 2018, 1086, 63–72. [Google Scholar] [CrossRef]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Nat. Prod. Res. 2008, 22, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zuccolotto, T.; Bressan, J.; Lourenço, A.V.F.; Bruginski, E.; Veiga, A.; Marinho, J.V.N.; Raeski, P.A.; Heiden, G.; Salvador, M.J.; Murakami, F.S.; et al. Chemical, Antioxidant, and Antimicrobial Evaluation of Essential Oils and an Anatomical Study of the Aerial Parts from Baccharis Species (Asteraceae). Chem. Biodivers. 2019, 16, e1800547. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (−)-T-Cadinol—A Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)—Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127. [Google Scholar] [CrossRef]
- Claeson, P.; Andersson, R.; Samuelsson, G. T-cadinol: A pharmacologically active constituent of scented myrrh: Introductory pharmacological characterization and high field 1H- and 13C-NMR data. Planta Med. 1991, 57, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tukashima, A.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Ōmura, S.; Yamada, H. In vitro antitrypanosomal activity of plant terpenes against Trypanosoma brucei. Phytochemistry 2011, 72, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, D.S.; Ferraz, R.P.C.; Carvalho, N.C.; Soares, M.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P. Eudesmol Isomers Induce Caspase-Mediated Apoptosis in Human Hepatocellular Carcinoma HepG2 Cells. Basic Clin. Pharmacol. Toxicol. 2013, 113, 300–306. [Google Scholar] [CrossRef]
- Asakura, K.; Kanemasa, T.; Minagawa, K.; Kagawa, K.; Yagami, T.; Nakajima, M.; Ninomiya, M. α-Eudesmol, a P/Q-type Ca2+ channel blocker, inhibits neurogenic vasodilation and extravasation following electrical stimulation of trigeminal ganglion. Brain Res. 2000, 873, 94–101. [Google Scholar] [CrossRef]
- Ohara, K.; Misaizu, A.; Kaneko, Y.; Fukuda, T.; Miyake, M.; Miura, Y.; Okamura, H.; Yajima, J.; Tsuda, A. β-Eudesmol, an Oxygenized Sesquiterpene, Reduces the Increase in Saliva 3-Methoxy-4-Hydroxyphenylglycol after the “Trier Social Stress Test” in Healthy Humans: A Randomized, Double-Blind, Placebo-Controlled Cross-Over Study. Nutrients 2018, 11, 9. [Google Scholar] [CrossRef]
- Tyagi, V.; Singh, V.K.; Sharma, P.K.; Singh, V. Essential oil-based nanostructures for inflammation and rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 101983. [Google Scholar] [CrossRef]
- Han, N.R.; Moon, P.D.; Ryu, K.J.; Jang, J.B.; Kim, H.M.; Jeong, H.J. β-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 257–265. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Ali, Z.; Baser, K.H.C.; Khan, I.A. Chemical Composition and Biological Activity of Four Salvia Essential Oils and Individual Compounds against Two Species of Mosquitoes. J. Agric. Food Chem. 2015, 63, 447–456. [Google Scholar] [CrossRef]
- Trevizan, L.N.F.; Nascimento, K.F.d.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.d.C.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Francke, W.; Schulz, S. Pheromones; Elsevier: Amsterdam, The Netherlands, 1999; pp. 197–261. [Google Scholar]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.; Guterres, Z.d.R.; Violante, I.M.P.; Lopes, T.F.S.; Garcez, W.S.; Garcez, F.R. Evaluation of mutagenic and antimicrobial properties of brown propolis essential oil from the Brazilian Cerrado biome. Toxicol. Rep. 2015, 2, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Beattie, K. Phytochemical Studies and Bioactivity of Centipeda and Eremophila Species; Southern Cross University: Lismore, NSW, Australia, 2009. [Google Scholar]
- Beattie, K.D.; Waterman, P.G.; Forster, P.I.; Thompson, D.R.; Leach, D.N. Chemical composition and cytotoxicity of oils and eremophilanes derived from various parts of Eremophila mitchellii Benth. (Myoporaceae). Phytochemistry 2011, 72, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Shinjo, Y.; Miyagi, M.; Matsuura, H.; Abe, T.; Kikuchi, N.; Suzuki, M. Investigation of insect repellent activity of cyclocolorenone obtained from the red alga Laurencia intricata. Rec. Nat. Prod. 2019, 13, 81–84. [Google Scholar] [CrossRef]
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980, 40, 403. [Google Scholar]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- Ito, M.; Shimura, H.; Watanabe, N.; Tamai, M.; Hanada, K.; Takahashi, A.; Tanaka, Y.; Arai, I.; Zhang, P.-L.; Rao, C.; et al. Hepatorotective Compounds from Canarium album and Euphorbia nematocypha. Chem. Pharm. Bull. 1990, 38, 2201–2203. [Google Scholar] [CrossRef]
- de Carvalho, N.C.; Neves, S.P.; Dias, R.B.; Valverde, L.d.F.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; dos Santos, E.R.; Oliveira, R.M.M.; Carlos, R.M.; et al. A novel ruthenium complex with xanthoxylin induces S-phase arrest and causes ERK1/2-mediated apoptosis in HepG2 cells through a p53-independent pathway. Cell Death Dis. 2018, 9, 79. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Yang, X.-N.; Zhu, X.; Xiao, X.-R.; Yang, X.-W.; Qin, H.-B.; Gonzalez, F.J.; Li, F. Role of Metabolic Activation in Elemicin-Induced Cellular Toxicity. J. Agric. Food Chem. 2019, 67, 8243–8252. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.T. Possible Implication of Myristicin as a Psychotropic Substance. Nature 1966, 210, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Purity, Antimicrobial Activities and Olfactory Evaluations of 2-Phenylethanol and Some Derivatives. J. Essent. Oil Res. 2008, 20, 82–85. [Google Scholar] [CrossRef]
- Chowdary, G.V.; Ramesh, M.N.; Prapulla, S.G. Enzymic synthesis of isoamyl isovalerate using immobilized lipase from Rhizomucor miehei: A multivariate analysis. Process Biochem. 2000, 36, 331–339. [Google Scholar] [CrossRef]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y.; Kunitomi, M.; Kudo, T.; MacDonald, P.N. Endocrine Disrupting Chemicals, Phthalic Acid and Nonylphenol, Activate Pregnane X Receptor-Mediated Transcription. Mol. Endocrinol. 2000, 14, 421–428. [Google Scholar] [CrossRef]
- Ahn, D.; Kwon, J.; Song, S.; Lee, J.; Yoon, S.; Chung, S.J. Methyl Syringate Stimulates Glucose Uptake by Inhibiting Protein Tyrosine Phosphatases Relevant to Insulin Resistance. Life 2023, 13, 1372. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, M.J.; Park, J.-H.; Ishii, S.; Misaka, T.; Rhyu, M.-R. Methyl syringate, a low-molecular-weight phenolic ester, as an activator of the chemosensory ion channel TRPA1. Arch. Pharmacal Res. 2012, 35, 2211–2218. [Google Scholar] [CrossRef]
- Phuong, N.T.; Cuong, T.T.; Quang, D.N. Anti-inflammatory activity of methyl ferulate isolated from Stemona tuberosa Lour. Asian Pac. J. Trop. Med. 2014, 7, S327–S331. [Google Scholar] [CrossRef]
- Sultana, R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim. Biophys. Acta 2012, 1822, 748–752. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Wang, Y.-Y.; Gao, Z.-Q.; Chen, D.; Liu, G.; Wan, B.-B.; Jiang, F.-J.; Wei, M.-X.; Zuo, J.; Zhu, J.; et al. Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 2069–2081. [Google Scholar] [CrossRef]
- Cunha, F.V.M.; Coelho, A.G.; Azevedo, P.S.d.S.; da Silva, A.A.; Oliveira, F.d.A.; Nunes, L.C.C. Systematic review and technological prospection: Ethyl ferulate, a phenylpropanoid with antioxidant and neuroprotective actions. Expert Opin. Ther. Pat. 2019, 29, 73–83. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Sharifi-Rad, M.; Marchetti, L.; Epifano, F.; Genovese, S. Auraptene and umbelliprenin: A review on their latest literature acquisitions. Phytochem. Rev. 2020, 21, 317–326. [Google Scholar] [CrossRef]
- Guan, Z.; Hellman, J.; Schumacher, M. Contemporary views on inflammatory pain mechanisms: TRPing over innate and microglial pathways. F1000Research 2016, 5, 2425. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Jeong, S.-G.; Park, J.-E.; Han, J.-A.; Kang, H.-R.; Lee, D.; Song, M.J. Antiviral activity of angelicin against gammaherpesviruses. Antivir. Res. 2013, 100, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Bhatti, R. Signaling Pathways Involved in the Neuroprotective Effect of Osthole: Evidence and Mechanisms. Mol. Neurobiol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jerris, P.J.; Smith, A.B. Synthesis and Configurational Assignment of Geiparvarin: A Novel Antitumor Agent. J. Org. Chem. 1981, 46, 577–585. [Google Scholar] [CrossRef]



| Species (Accepted Name) | Synonyms |
|---|---|
| Geijera balansae (Baill.) Schinz & Guillaumin | Zanthoxylum balansae |
| Geijera cauliflora Baill. | Dendrosma deplanchei Pancher & Sebert Geijera deplanchei (Pancher & Sebert) Däniker Geijera lateriflora Baill. ex Guillaumin |
| Geijera linearifolia (DC.) J.M.Black | Geijera parviflora var. crassifolia Benth. Eriostemon linearifolius DC. Geijera linearifolia Domin |
| Geijera parviflora Lindl. | Geijera pendula Lindl. Geijera parviflora var. parviflora Lindl. Zanthoxylum australasicum A.Juss. |
| Geijera salicifolia Schott | Geijera salicifolia var. augustifolia Maiden Geijera salicifolia Schott var. salicifolia Geijera salicifolia var. latifolia (Lindl.) Domin Geijera salicifolia var. angustifolia Maiden & Betche Geijera latifolia Lindl. Geijera salicifolia var. typica Domin Geijera floribunda Pancher ex Guillaumin |
| Geijera tartarea T.G.Hartley ex Munzinger & Bruy | None |
| Type of Activity | No. Compounds in Geijera | No. Compounds in G. balansae | No. Compounds in G. parviflora | No. Compounds in G. salicifolia | No. Compounds in G. linearifolia |
|---|---|---|---|---|---|
| Acetylcholinesterase inhibition | 7 | 1 | 6 | 6 | - |
| Anti-cancer | 41 | 4 | 32 | 26 | 13 |
| Anticonvulsant | 4 | 1 | 3 | 3 | 2 |
| Antifungal | 25 | 5 | 16 | 13 | 9 |
| Antimicrobial | 45 | 9 | 29 | 19 | 12 |
| Antioxidant | 20 | 4 | 15 | 11 | 2 |
| Increase in membrane permeability | 3 | - | 2 | 3 | - |
| Monoamine oxidase B inhibition | 1 | - | 1 | 1 | - |
| Muscle relaxant | 5 | 2 | 2 | 3 | 1 |
| Osteogenic | 3 | 1 | 2 | - | - |
| Plant pest resistance/semiochemical/ insecticide | 26 | 1 | 21 | 14 | 9 |
| Psychoactive | 3 | - | 3 | 2 | - |
| Reduction in anxiety | 7 | - | 5 | 5 | 2 |
| Reduction in inflammation | 38 | 7 | 28 | 17 | 6 |
| Reduction in pain | 12 | 1 | 8 | 10 | 3 |
| umbelliferone 1 S | xanthoxyletin 19 B | sabinene 52 P,S | β-caryophyllene 83 P,S,L |
| 6′-dehydromarmin 5 P | dictamine 25 B | α-phellandrene 53 P | (E,E)-α-farnesene 86 P,S,L |
| (R)-6-O-(4-geranyloxy-2-hydroxy) cinnamoylmarmin 7 P | skimmianine 26 S,B | citronellyl acetate 56 L | α-eudesmol 95 P,S,L |
| parvifloranine A 8 P | flindersine 30 P,B | linalool 61 P,S,L | β-eudesmol 96 P,S,L |
| scoparone 11 P | N-(acetoxymethyl) flindersine 35 P | α-terpineol 62 P,S | viridiflorol 98 P |
| suberosin 12 P | haplaphine 36 P,B | terpinen-4-ol 63 P,S | caryophyllene oxide 100 P,S,L |
| dehydrogeijerin 13 P,S | myrcene 44 P,S | 1,8 cineole 64 P,S | spathulenol 102 P,S,L |
| 6-(methoxyl) geiparvarin 14 P | γ-terpinene 47 P,S | camphor 65 P,S | cyclocolorenone 104 P |
| osthole 15 P | α-pinene 49 P,S | borneol 66 S | brevifolin (xanthoxylin) 106 P,S,B |
| angelicin (isopsoralen) 16 P | β-pinene 50 P,S | azulene 67 P | methyl ferulate 116 B |
| luvangetin 18 B | p-cymene 55 P,S | α-caryophyllene (humulene) 82 S | ethyl ferulate 117 B |
| umbelliferone 1 S | zanthobungeanine 33 B | nerol 59 L | guaiol 88 P,S |
| auraptene 3 P | 4-methoxy N-methyl-2-quinolone 37 B | geraniol 60 L | elemol 89 P,S |
| scoparone 11 P | hordenine 41 B | linalool 61 P,S,L | ledol 91 P |
| osthole 15 P | (E)-β-ocimene 42 P,S,L | α-terpineol 62 P,S | globulol 92 P |
| angelicin (isopsoralen) 16 P | (Z)-β-ocimene 43 P,S,L | terpinen-4-ol 63 P,S | epi-globulol 93 P |
| xanthyletine 17 P | myrcene 44 P,S | 1,8 cineole 64 P,S | τ-cadinol 94 L |
| luvangetin 18 B | α-terpinene 46 P,S | camphor 65 P,S | α-eudesmol 95 P,S,L |
| xanthoxyletin 19 B | γ-terpinene 47 P,S | borneol 66 S | viridiflorol 98 P |
| 11′-hexadecanoyl anthranillic acid 20 P | terpinolene 48 P,S | viridiflorene (ledene) 71 L | spathulenol 102 P,S,L |
| 9′-hexadecenoyl anthranillic acid 21 P | camphene 51 P | germacrene B 75 S | cyclocolorenone 104 P |
| 7′-hexadecanoyl anthranillic acid 22 P | sabinene 52 P,S | germacrene D 76 P,S,L | brevifolin (xanthoxylin) 106 P,S,B |
| hexadecanoyl anthranillic acid 24 P | α-phellandrene 53 P | α-caryophyllene (humulene) 82 S | elemicin 107 P |
| dictamnine 25 B | β-phellandrene 54 P,S | β-caryophyllene 83 P,S,L | ethyl ferulate 117 B |
| γ-fagarine 27 S,B | p-cymene 53 P,S | aromadendrene 85 P,S,L | |
| platydesmine 28 S,B | citronellyl acetate 54 L | (E,E)-α-farnesene 86 P,S,L | |
| flindersine 30 P,B | geranyl acetate 57 L | (E,E)-farnesol 87 L |
| Acetylcholinesterase Inhibitors | Anxiolytics and Sedatives | Muscle Relaxants and Anticonvulsants | Psychoactive Compounds |
|---|---|---|---|
| geijerin 10 P,S | osthole 15 P | xanthoxyletin 19 B | geiparvarin 2 P,S |
| dehydrogeijerin 13 P,S | myrcene 44 P,S | geibalansine 38 B | myrcene 44 P,S |
| skimmianine 26 S,B | limonene 45 S | (E)-β-ocimene 42 P,S,L | elemicin 107 P |
| α-terpinene 46 P,S | α-terpinene 46 P,S | (Z)-β-ocimene 43 P,S,L | |
| γ-terpinene 47 P,S | linalool 61 P,S,L | myrcene 44 P,S | |
| β-phellandrene 54 P,S | borneol 66 S | α-terpineol 62 P,S | |
| elemicin 107 P | β-eudesmol 96 P,S,L | borneol 66 S | |
| τ-cadinol 94 L | |||
| brevifolin (xanthoxylin) 106 P,S |
| umbelliferone 1 S | dictamnine 25 B | p-cymene 55 P,S | β-caryophyllene 83 P,S,L |
| geiparvarin 2 P,S | skimmianine 26 S, B | citronellyl acetate 56 L | (E,E)-α-farnesene 86 P,S,L |
| auraptene 3 P | haplaphine 36 P,B | geranyl acetate 57 L | guaiol 88 P,S |
| 6′dehydromarmin 5 P | (E)-β-ocimene 42 P,S,L | α-terpineol 62 P,S | α-eudesmol 95 P,S,L |
| 2′,3′-dihydrogeiparvarin 6 P,S | (Z)-β-ocimene 43 P,S,L | terpinen-4-ol 63 P,S | β-eudesmol 96 P,S,L |
| (R)-6-O-(4-geranyloxy-2-hydroxy) cinnamoylmarmin 7 P | myrcene 44 P,S | camphor 65 P,S | γ-eudesmol 97 P,S,L |
| scoparone 11 P | limonene 45 S | germacrene D 76 P,S,L | caryophyllene oxide 100 P,S,L |
| 6-(methoxyl) geiparvarin 14 P | α-pinene 49 P,S | δ-cadinene 79 P | spathulenol 102 P,S,L |
| osthole 15 P | β-pinene 50 P,S | β-elemene 80 P,S,L | eremophilone 103 P |
| angelicin (isopsoralen) 16 P | α-phellandrene 53 P | α-caryophyllene (humulene) 82 S | β-sitosterol 105 S |
| xanthoxyletin 19 B |
| Insecticides | Semiochemicals | Antifeedants | Oviposition Deterrents |
|---|---|---|---|
| terpinolene 48 P,S | (E)-β-ocimene 42 P,S,L | umbelliferone 1 S | pregeijerene 68 S |
| α-phellandrene 53 P | (Z)-β-ocimene 43 P,S,L | hordenine 41 B | geijerene 70 S,P |
| citronellyl acetate 56 L | α-santalene 84 P | pregeijerene 68 S | |
| α-terpineol 62 P,S | (E,E)-α-farnesene 86 P,S,L | geijerene 70 S,P | |
| camphor 65 P,S | palustrol 90 L | α-bergamotene 78 P | |
| germacrene D 73 P,S,L | epi-globulol 93 P | eremophilone 103 P | |
| bicyclogermacrene 77 P,S,L | β-eudesmol 96 P,S,L | cyclocolorenone 104 P | |
| δ-cadinene 79 P | (E,E)-farnesal 99 L | ||
| γ-elemene 81 P,S | cis-jasmone 111 P | ||
| guaiol 88 P,S | methyl eugenol 112 P | ||
| β-eudesmol 96 P,S,L | |||
| eremophilone 103 P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugan, D.; Bell, R.J.; Brkljača, R.; Rix, C.; Urban, S. A Review of the Ethnobotanical Use, Chemistry and Pharmacological Activities of Constituents Derived from the Plant Genus Geijera (Rutaceae). Metabolites 2024, 14, 81. https://doi.org/10.3390/metabo14020081
Dugan D, Bell RJ, Brkljača R, Rix C, Urban S. A Review of the Ethnobotanical Use, Chemistry and Pharmacological Activities of Constituents Derived from the Plant Genus Geijera (Rutaceae). Metabolites. 2024; 14(2):81. https://doi.org/10.3390/metabo14020081
Chicago/Turabian StyleDugan, Deepika, Rachael J. Bell, Robert Brkljača, Colin Rix, and Sylvia Urban. 2024. "A Review of the Ethnobotanical Use, Chemistry and Pharmacological Activities of Constituents Derived from the Plant Genus Geijera (Rutaceae)" Metabolites 14, no. 2: 81. https://doi.org/10.3390/metabo14020081