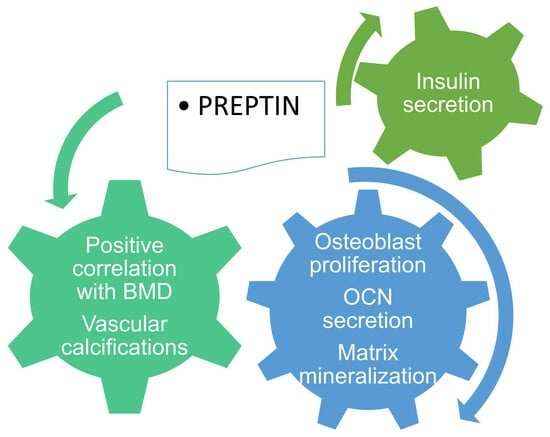
Preptin: A New Bone Metabolic Parameter?
Abstract
:1. Introduction
2. Methods
3. Preptin, Insulin Resistance and Bone Metabolism
3.1. Insulin Resistance: The Contradictory Status of Preptin in PCOS
3.2. The Dual Role of Insulin Resistance in Bone Mass and Metabolism
4. The Impact of Preptin on Bone and Calcium Metabolism
4.1. What Do We Know from Experimental Data
4.2. Preptin and Bone Mass and Metabolism in The Clinical Setting
| Research | Study Group | BMI (kg/m2) | Preptin Concentration | Outcome |
|---|---|---|---|---|
| Preptin and BMD | ||||
| Li et al. [64] 2013 | 52 elderly men with osteoporosis | 22.34 ± 1.84 * | 5.1 ± 0.69 ng/mL * | Preptin (whole-group):
|
| 52 elderly men with osteopenia | 22.79 ± 1.29 * | 7.09 ± 1.36 ng/mL * | ||
| 31 aged-matched controls | 22.42 ± 1.55 * | 10.11 ± 1.61 ng/mL * | ||
| Aahmad et al. [76] 2018 | 30 preM women | 24.58 ± 4.4 * | 2667.3 ± 940.41 ng/L * | Preptin (whole-group):
|
| 30 postM women | 24.95 ± 3.15 * | 2102.27 ± 918.66 ng/L * | ||
| Kaluzna et al. [77] 2021 | 36 HD + DM/IGT | 27.1 (4.9) ** | 512 (1030.50) ng/L ** | Preptin (whole group):
|
| 37 HD + NGT | 23.3 (5.5) ** | 595 (788) ng/L ** | ||
| Preptin and bone and calcium metabolism | ||||
| El-Eshmawy et al. [57] 2015 | 50 overweight | 27.5 ± 1.48 * | 484.2 ± 50.84 pg/mL * | Preptin (overweight + obese group):
|
| 50 obese | 33.3 ± 2 * | 516.5 ± 66.98 pg/mL * | ||
| 50 controls | 23.9 ± 0.57 * | 366.4 ± 38.53 pg/mL * | ||
| Li et al. [78] 2018 | 102 non-CAC patients | 25.83 ± 4.28 * | 9.5 ± 3.91 ng/mL * | Preptin:
|
| 118 CAC patients | 25.42 ± 4.25 * | 11.59 ± 7.81 ng/mL * | ||
| Bebars et al. [79] 2019 | 30 rachitic children | NA | 6.3 ± 1.5 ng/L * | Preptin (rachitic children):
|
| 30 non-rachitic children | NA | 8.3 ± 1.8 ng/L * | ||
4.3. Preptin: A New Player in Vascular Calcifications
4.4. Preptin and Variants of Abnormal Bone Metabolism
5. Preptin in Osteoporosis—Therapeutical Challenges
6. Future Directions: Bone Cancer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aydin, S. Three New Players in Energy Regulation: Preptin, Adropin and Irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Phillips, A.R.J.; Cooper, G.J.S. Preptin Derived from Proinsulin-like Growth Factor II (ProIGF-II) Is Secreted from Pancreatic Islet Beta-Cells and Enhances Insulin Secretion. Biochem. J. 2001, 360, 431–439. [Google Scholar] [CrossRef]
- Cheng, K.C.; Li, Y.X.; Asakawa, A.; Ushikai, M.; Kato, I.; Sato, Y.; Cheng, J.T.; Inui, A. Characterization of Preptin-Induced Insulin Secretion in Pancreatic β-Cells. J. Endocrinol. 2012, 215, 43–49. [Google Scholar] [CrossRef]
- Buckels, E.J.; Hsu, H.L.; Buchanan, C.M.; Matthews, B.G.; Lee, K.L. Genetic Ablation of the Preptin-Coding Portion of Igf2 Impairs Pancreatic Function in Female Mice. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E467–E479. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Chen, W.; Liu, H.; Boden, G.; Li, K. Circulating Preptin Levels in Normal, Impaired Glucose Tolerance, and Type 2 Diabetic Subjects. Ann. Med. 2009, 41, 52–56. [Google Scholar] [CrossRef]
- Celik, O.; Celik, N.; Hascalik, S.; Sahin, I.; Aydin, S.; Ozerol, E. An Appraisal of Serum Preptin Levels in PCOS. Fertil. Steril. 2011, 95, 314–316. [Google Scholar] [CrossRef]
- Aslan, M.; Celik, O.; Karsavuran, N.; Celik, N.; Dogan, D.G.; Botan, E.; Kafkasli, A. Maternal Serum and Cord Blood Preptin Levels in Gestational Diabetes Mellitus. J. Perinatol. 2010, 31, 350–355. [Google Scholar] [CrossRef]
- Reid, I.R. Fat and Bone. Arch. Biochem. Biophys. 2010, 503, 20–27. [Google Scholar] [CrossRef]
- Naot, D.; Cornish, J. Cytokines and Hormones That Contribute to the Positive Association between Fat and Bone. Front. Endocrinol. 2014, 5, 70. [Google Scholar] [CrossRef]
- Zhang, M.; Xuan, S.; Bouxsein, M.L.; Von Stechow, D.; Akeno, N.; Faugere, M.C.; Malluche, H.; Zhao, G.; Rosen, C.J.; Efstratiadis, A.; et al. Osteoblast-Specific Knockout of the Insulin-like Growth Factor (IGF) Receptor Gene Reveals an Essential Role of IGF Signaling in Bone Matrix Mineralization. J. Biol. Chem. 2002, 277, 44005–44012. [Google Scholar] [CrossRef]
- Livingstone, C.; Borai, A. Insulin-like Growth Factor-II: Its Role in Metabolic and Endocrine Disease. Clin. Endocrinol. 2014, 80, 773–781. [Google Scholar] [CrossRef]
- Bu, Z.; Kuok, K.; Meng, J.; Wang, R.; Xu, B.; Zhang, H. The Relationship between Polycystic Ovary Syndrome, Glucose Tolerance Status and Serum Preptin Level. Reprod. Biol. Endocrinol. 2012, 10, 10. [Google Scholar] [CrossRef]
- Wang, R.; Xue, A.; Zheng, W.; Wang, L.; Yan, F.; Hu, W.; Lin, J.; He, L. Elevated Serum Preptin Concentrations in Patients with Diabetic Nephropathy. J. Investig. Med. 2019, 67, 1048–1052. [Google Scholar] [CrossRef]
- Güngör Kobat, S.; Gül, F.C.; Çelik, F.; Liman Uzun, S.; Kobat, M.A.; Akkoç, R.F.; Aydın, S. Plasma and Aqueous Levels of Subfatin, Preptin and Betatrophin in Patients with Diabetic Retinopathy. BMC Ophthalmol. 2023, 23, 312. [Google Scholar] [CrossRef]
- Kruszewska, J.; Laudy-Wiaderny, H.; Kunicki, M. Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients-The Debate Is Still Open. Int. J. Environ. Res. Public Health 2022, 19, 2099. [Google Scholar] [CrossRef]
- Şentürk, Ş.; Hatirnaz, S.; Kanat-Pektaş, M. Serum Preptin and Amylin Levels with Respect to Body Mass Index in Polycystic Ovary Syndrome Patients. Med. Sci. Monit. 2018, 24, 7517. [Google Scholar] [CrossRef]
- Mierzwicka, A.; Kuliczkowska-Plaksej, J.; Kolačkov, K.; Bolanowski, M. Preptin in Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2018, 34, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Bahreiny, S.S.; Harooni, E.; Dabbagh, M.R.; Ebrahimi, R. Circulating Serum Preptin Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Reprod. Biomed. 2023, 21, 367–378. [Google Scholar] [CrossRef]
- Ozkan, Y.; Timurkan, E.S.; Aydin, S.; Sahin, I.; Timurkan, M.; Citil, C.; Kalayci, M.; Yilmaz, M.; Aksoy, A.; Catak, Z. Acylated and Desacylated Ghrelin, Preptin, Leptin, and Nesfatin-1 Peptide Changes Related to the Body Mass Index. Int. J. Endocrinol. 2013, 2013, 236085. [Google Scholar] [CrossRef] [PubMed]
- Safarimosavi, S.; Mohebbi, H.; Rohani, H. High-Intensity Interval vs. Continuous Endurance Training: Preventive Effects on Hormonal Changes and Physiological Adaptations in Prediabetes Patients. J. Strength Cond. Res. 2021, 35, 731–738. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Wang, W.; Liu, J. Insulin Stimulates Osteoblast Proliferation and Differentiation through ERK and PI3K in MG-63 Cells. Cell Biochem. Funct. 2010, 28, 334–341. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.; Wan, C.; Chen, D.; Faugere, M.C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin Receptor Signaling in Osteoblasts Regulates Postnatal Bone Acquisition and Body Composition. Cell 2010, 142, 309. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin Signaling in Osteoblasts Integrates Bone Remodeling and Energy Metabolism. Cell 2010, 142, 296. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Epstein, S.; Napoli, N. Insulin Resistance and Bone: A Biological Partnership. Acta Diabetol. 2018, 55, 305–314. [Google Scholar] [CrossRef]
- Aguiari, P.; Leo, S.; Zavan, B.; Vindigni, V.; Rimessi, A.; Bianchi, K.; Franzin, C.; Cortivo, R.; Rossato, M.; Vettor, R.; et al. High Glucose Induces Adipogenic Differentiation of Muscle-Derived Stem Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1226–1231. [Google Scholar] [CrossRef]
- Shanbhogue, V.V.; Mitchell, D.M.; Rosen, C.J.; Bouxsein, M.L. Type 2 Diabetes and the Skeleton: New Insights into Sweet Bones. Lancet Diabetes Endocrinol. 2016, 4, 159–173. [Google Scholar] [CrossRef]
- Starup-Linde, J.; Hygum, K.; Harsløf, T.; Langdahl, B. Type 1 Diabetes and Bone Fragility: Links and Risks. Diabetes Metab. Syndr. Obes. 2019, 12, 2539–2547. [Google Scholar] [CrossRef]
- Starup-Linde, J.; Lykkeboe, S.; Gregersen, S.; Hauge, E.M.; Langdahl, B.L.; Handberg, A.; Vestergaard, P. Bone Structure and Predictors of Fracture in Type 1 and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 928–936. [Google Scholar] [CrossRef]
- Delbari, N.; Rajaei, A.; Oroei, M.; Ahmadzadeh, A.; Farsad, F. A Comparison between Femoral Neck and LS-BMD with LS-TBS in T2DM Patients: A Case Control Study. BMC Musculoskelet. Disord. 2021, 22, 582. [Google Scholar] [CrossRef]
- Hariri, A.F.; Almatrafi, M.N.; Zamka, A.B.; Babaker, A.S.; Fallatah, T.M.; Althouwaibi, O.H.; Hamdi, A.S. Relationship between Body Mass Index and T -Scores of Bone Mineral Density in the Hip and Spine Regions among Older Adults with Diabetes: A Retrospective Review. J. Obes. 2019, 2019, 9827403. [Google Scholar] [CrossRef]
- Bilha, S.C.; Leustean, L.; Preda, C.; Branisteanu, D.D.; Mihalache, L.; Ungureanu, M.C. Bone Mineral Density Predictors in Long-Standing Type 1 and Type 2 Diabetes Mellitus. BMC Endocr. Disord. 2021, 21, 156. [Google Scholar] [CrossRef]
- Kasperk, C.; Georgescu, C.; Nawroth, P. Diabetes Mellitus and Bone Metabolism. Exp. Clin. Endocrinol. Diabetes 2017, 125, 213–217. [Google Scholar] [CrossRef]
- Wei, J.; Ferron, M.; Clarke, C.J.; Hannun, Y.A.; Jiang, H.; Blaner, W.S.; Karsenty, G. Bone-Specific Insulin Resistance Disrupts Whole-Body Glucose Homeostasis via Decreased Osteocalcin Activation. J. Clin. Investig. 2014, 124, 1781–1793. [Google Scholar] [CrossRef]
- Shanbhogue, V.V.; Finkelstein, J.S.; Bouxsein, M.L.; Yu, E.W. Association between Insulin Resistance and Bone Structure in Nondiabetic Postmenopausal Women. J. Clin. Endocrinol. Metab. 2016, 101, 3114–3122. [Google Scholar] [CrossRef]
- Napoli, N.; Conte, C.; Pedone, C.; Strotmeyer, E.S.; Barbour, K.E.; Black, D.M.; Samelson, E.J.; Schwartz, A.V. Effect of Insulin Resistance on BMD and Fracture Risk in Older Adults. J. Clin. Endocrinol. Metab. 2019, 104, 3303–3310. [Google Scholar] [CrossRef]
- Yüksel, O.; Dökmetaş, H.S.; Topcu, S.; Erselcan, T.; Şencan, M. Relationship between Bone Mineral Density and Insulin Resistance in Polycystic Ovary Syndrome. J. Bone Miner. Metab. 2001, 19, 257–262. [Google Scholar] [CrossRef]
- Noyan, V.; Yucel, A.; Sagsoz, N. The Association of Bone Mineral Density with Insulin Resistance in Patients with Polycystic Ovary Syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 200–205. [Google Scholar] [CrossRef]
- Verroken, C.; Zmierczak, H.G.; Goemaere, S.; Kaufman, J.M.; Lapauw, B. Insulin Resistance Is Associated with Smaller Cortical Bone Size in Nondiabetic Men at the Age of Peak Bone Mass. J. Clin. Endocrinol. Metab. 2017, 102, 1807–1815. [Google Scholar] [CrossRef]
- Viljakainen, H.; Ivaska, K.K.; Paldánius, P.; Lipsanen-Nyman, M.; Saukkonen, T.; Pietiläinen, K.H.; Andersson, S.; Laitinen, K.; Mäkitie, O. Suppressed Bone Turnover in Obesity: A Link to Energy Metabolism? A Case-Control Study. J. Clin. Endocrinol. Metab. 2014, 99, 2155–2163. [Google Scholar] [CrossRef]
- Xiao, C.; Li, W.; Lu, T.; Wang, J.; Han, J. Preptin Promotes Proliferation and Osteogenesis of MC3T3-E1 Cells by Upregulating β-Catenin Expression. IUBMB Life 2019, 71, 854–862. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.N.; Komm, B.S.; Javed, A.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT Signaling Promotes Osteogenesis by Directly Stimulating Runx2 Gene Expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Haxaire, C.; Haÿ, E.; Geoffroy, V. Runx2 Controls Bone Resorption through the Down-Regulation of the Wnt Pathway in Osteoblasts. Am. J. Pathol. 2016, 186, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Bonewald, L.F. The Role of the Wnt/β-Catenin Signaling Pathway in Formation and Maintenance of Bone and Teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/β-Catenin Signaling Is Required for Normal Bone Homeostasis. Mol. Cell Biol. 2010, 30, 3071. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.A.; Picconi, J.L.; Lara-Castillo, N.; Johnson, M.L. Activation of β–Catenin Signaling in MLO-Y4 Osteocytic Cells versus 2T3 Osteoblastic Cells by Fluid Flow Shear Stress and PGE2: Implications for the Study of Mechanosensation in Bone. Bone 2010, 47, 872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Z.; Yu, Y.; Chu, H.Y.; Yu, S.; Yao, S.; Zhang, G.; Zhang, B.T. Drug Discovery of DKK1 Inhibitors. Front. Pharmacol. 2022, 13, 847387. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Bava, U.; Watson, M.; Xu, X.; Lin, J.M.; Chan, V.A.; Grey, A.B.; Naot, D.; Buchanan, C.M.; et al. Preptin, Another Peptide Product of the Pancreatic β-Cell, Is Osteogenic in Vitro and in Vivo. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lenormand, P.; Brondello, J.M.; Brunet, A.; Pouysségur, J. Growth Factor–Induced P42/P44 MAPK Nuclear Translocation and Retention Requires Both MAPK Activation and Neosynthesis of Nuclear Anchoring Proteins. J. Cell Biol. 1998, 142, 625. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, Y.S.; Park, K.H.; Oh, H.; Greenblatt, M.B.; Shim, J.H. The ERK MAPK Pathway Is Essential for Skeletal Development and Homeostasis. Int. J. Mol. Sci. 2019, 20, 1803. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Carballo, E.; Gamez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Bikkavilli, R.K.; Malbon, C.C. Mitogen-Activated Protein Kinases and Wnt/β-Catenin Signaling: Molecular Conversations among Signaling Pathways. Commun. Integr. Biol. 2009, 2, 46. [Google Scholar] [CrossRef]
- Liu, Y.S.; Lu, Y.; Liu, W.; Xie, H.; Luo, X.H.; Wu, X.P.; Yuan, L.Q.; Liao, E.Y. Connective Tissue Growth Factor Is a Downstream Mediator for Preptin-Induced Proliferation and Differentiation in Human Osteoblasts. Amino Acids 2010, 38, 763–769. [Google Scholar] [CrossRef]
- Safadi, F.F.; Xu, J.; Smock, S.L.; Kanaan, R.A.; Selim, A.H.; Odgren, P.R.; Marks, S.C.; Owen, T.A.; Popoff, S.N. Expression of Connective Tissue Growth Factor in Bone: Its Role in Osteoblast Proliferation and Differentiation in Vitro and Bone Formation in Vivo. J. Cell Physiol. 2003, 196, 51–62. [Google Scholar] [CrossRef]
- Hendesi, H.; Barbe, M.F.; Safadi, F.F.; Monroy, M.A.; Popoff, S.N. Integrin Mediated Adhesion of Osteoblasts to Connective Tissue Growth Factor (CTGF/CCN2) Induces Cytoskeleton Reorganization and Cell Differentiation. PLoS ONE 2015, 10, e0115325. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah Abulfadle, K.; Refaat Abdelkader Atia, R.; Osama Mohammed, H.; Saad Ramadan, R.; Mohammed, N.A. The Potential Anti-Osteoporotic Effect of Exercise—Induced Increased Preptin Level in Ovariectomized Rats. Anat. Sci. Int. 2023, 98, 22–35. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Podufaly, A.; Dillon, P.F. Estradiol Binds to Insulin and Insulin Receptor Decreasing Insulin Binding in Vitro. Front. Endocrinol. 2014, 5, 98586. [Google Scholar] [CrossRef] [PubMed]
- El-Eshmawy, M.; Aal, I.A. Relationships between Preptin and Osteocalcin in Obese, Overweight, and Normal Weight Adults. Appl. Physiol. Nutr. Metab. 2015, 40, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases. Front. Physiol. 2021, 12, 692642. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Arisha, A.H. Swim Therapy-Induced Tissue Specific Metabolic Responses in Male Rats. Life Sci. 2020, 262, 118516. [Google Scholar] [CrossRef] [PubMed]
- Mogulkoc, R.; Dasdelen, D.; Baltaci, S.B.; Baltaci, A.K.; Sivrikaya, A. The Effect of Thyroid Dysfunction and Treatment on Adropin, Asprosin and Preptin Levels in Rats. Horm. Mol. Biol. Clin. Investig. 2020, 42, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, M.K.; Kwak, M.K.; Kim, D.; Hong, E.G. Association between Thyroid Hormones and Insulin Resistance Indices Based on the Korean National Health and Nutrition Examination Survey. Sci. Rep. 2021, 11, 21738. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Sabbatini, M.; Nicolì, E.; Fusaro, L.; Cannas, M. Effects and Differentiation Activity of IGF-I, IGF-II, Insulin and Preptin on Human Primary Bone Cells. Growth Factors 2013, 31, 57–65. [Google Scholar] [CrossRef]
- Bortolin, R.H.; Freire Neto, F.P.; Arcaro, C.A.; Bezerra, J.F.; da Silva, F.S.; Ururahy, M.A.; Souza, K.S.; Lima, V.M.; Luchessi, A.D.; Lima, F.P.; et al. Anabolic Effect of Insulin Therapy on the Bone: Osteoprotegerin and Osteocalcin Up-Regulation in Streptozotocin-Induced Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2016, 120, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y.B.; Han, J.; Liang, W.; Wang, J.Y.; Zhou, J.R.; Shen, Y.; Zhang, J. Lower Circulating Preptin Levels in Male Patients with Osteoporosis Are Correlated with Bone Mineral Density and Bone Formation. BMC Musculoskelet. Disord. 2013, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Liang, S.; Wu, Z.; Chen, Z.; Hu, L.; Qian, A. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2020, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Polgreen, L.E.; Jacobs, D.R.; Nathan, B.M.; Steinberger, J.; Moran, A.; Sinaiko, A.R. Association of Osteocalcin with Obesity, Insulin Resistance, and Cardiovascular Risk Factors in Young Adults. Obesity 2012, 20, 2194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, B.; Xu, Y.; Xu, H.; Zhang, N. The Relationship between Serum Osteocalcin Concentration and Glucose Metabolism in Patients with Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2013, 2013, 842598. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Arrieta, F.; Piñera, M.; Botella-Carretero, J.I.; Balsa, J.A.; Zamarrõn, I.; Menacho, M.; Díez, J.J.; Muñoz, T.; Vázquez, C. Serum Concentrations of Osteocalcin, Procollagen Type 1 N-Terminal Propeptide and Beta-CrossLaps in Obese Subjects with Varying Degrees of Glucose Tolerance. Clin. Endocrinol. 2011, 75, 184–188. [Google Scholar] [CrossRef]
- Kocak, T.; Tek, N.A. Osteocalcin: A New Phenomenon for Type 2 Diabetes and Obesity. Eur. J. Environ. Public Health 2023, 2023, 2542–4904. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P. The Role of Osteocalcin in the Endocrine Cross-Talk between Bone Remodelling and Energy Metabolism. Diabetologia 2011, 54, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic Nervous System in Obesity and Insulin-Resistance—The Complex Interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New Insights into the Biology of Osteocalcin. Bone 2016, 82, 42. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.M.d.S.; Masquio, D.C.L.; Corgosinho, F.C.; de Carvalho-Ferreira, J.P.; Netto, B.D.M.; Clemente, A.P.G.; Tock, L.; Tufik, S.; de Mello, M.T.; Dâmaso, A.R. Relationship between Adiponectin and Leptin on Osteocalcin in Obese Adolescents during Weight Loss Therapy. Arch. Endocrinol. Metab. 2018, 62, 275. [Google Scholar] [CrossRef] [PubMed]
- Motyl, K.J.; Rosen, C.J. Understanding Leptin-Dependent Regulation of Skeletal Homeostasis. Biochimie 2012, 94, 2089. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Nazari Soltan Aahmad, S.; Nourollahi, S.; Kazerouni, F.; Kianmehr, N.; Hajipour, H.; Sanajou, D.; Hosseini, V. Investigation of the Relation between Bone Mass Density and Serum Preptin Levels in Pre- and Postmenopausal Women. J. Bone Miner. Metab. 2018, 36, 710–715. [Google Scholar] [CrossRef]
- Kałużna, M.; Pawlaczyk, K.; Schwermer, K.; Hoppe, K.; Ibrahim, A.Y.; Czlapka-Matyasik, M.; Wrotkowska, E.; Ziemnicka, K.; Oko, A.; Ruchała, M. Is Preptin a New Bone Metabolism Parameter in Hemodialysis Patients? Life 2021, 11, 341. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Zhang, T.; Song, L.; Lei, C.; Zhao, Y.; He, B.; Zhao, Y.; Yin, B.; Jin, X.; et al. Preptin Is a New Predictor of Coronary Artery Calcification. Clin. Chim. Acta 2018, 485, 133–138. [Google Scholar] [CrossRef]
- Bebars, G.M.; Sallam, S.A.; Gaber, S.S.; Abdelaziz, A.H. Assessment of Preptin Peptide Level in the Sera of Rachitic Children and in Breast Milk of Their Mothers. Ital. J. Pediatr. 2019, 45, 34. [Google Scholar] [CrossRef]
- Thacher, T.D.; Fischer, P.R.; Pettifor, J.M. The Effect of Nutritional Rickets on Bone Mineral Density. J. Clin. Endocrinol. Metab. 2014, 99, 4174–4180. [Google Scholar] [CrossRef]
- Guthoff, M.; Wagner, R.; Vosseler, D.; Peter, A.; Nadalin, S.; Häring, H.U.; Fritsche, A.; Heyne, N. Impact of End-Stage Renal Disease on Glucose Metabolism—A Matched Cohort Analysis. Nephrol. Dial. Transplant. 2017, 32, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Napoletano, A.; Provenzano, M.; Garofalo, C.; Bini, C.; Comai, G.; La Manna, G. Mineral Bone Disorders in Kidney Disease Patients: The Ever-Current Topic. Int. J. Mol. Sci. 2022, 23, 12223. [Google Scholar] [CrossRef] [PubMed]
- Slouma, M.; Sahli, H.; Bahlous, A.; Laadhar, L.; Smaoui, W.; Rekik, S.; Gharsallah, I.; Sallami, M.; Moussa, F.B.; Elleuch, M.; et al. Mineral Bone Disorder and Osteoporosis in Hemodialysis Patients. Adv. Rheumatol. 2020, 60, 15. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [CrossRef]
- Bover, J.; Aguilar, A.; Arana, C.; Molina, P.; Lloret, M.J.; Ochoa, J.; Berná, G.; Gutiérrez-Maza, Y.G.; Rodrigues, N.; D’Marco, L.; et al. Clinical Approach to Vascular Calcification in Patients with Non-Dialysis Dependent Chronic Kidney Disease: Mineral-Bone Disorder-Related Aspects. Front. Med. 2021, 8, 642718. [Google Scholar] [CrossRef]
- Rashdan, N.A.; Sim, A.M.; Cui, L.; Phadwal, K.; Roberts, F.L.; Carter, R.; Ozdemir, D.D.; Hohenstein, P.; Hung, J.; Kaczynski, J.; et al. Osteocalcin Regulates Arterial Calcification Via Altered Wnt Signaling and Glucose Metabolism. J. Bone Miner. Res. 2020, 35, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; John Ballard, F.; Conover, C.A. Use of Site-Specific Antibodies to Characterize the Circulating Form of Big Insulin-like Growth Factor II in Patients with Hepatitis C-Associated Osteosclerosis. J. Clin. Endocrinol. Metab. 2002, 87, 3867–3870. [Google Scholar] [CrossRef]
- de Camargo Vieira, M.C.; Gonçalves, W.R.B.; Guerra, R.A.; Callegaro, F.S.; Lazaretti-Castro, M.; Maeda, S.S. Hepatitis C-Associated Osteosclerosis: Improvement After Treatment with Sofosbuvir, Daclatasvir, and Ibandronate: Case Report and Literature Review. Calcif. Tissue Int. 2021, 109, 104–109. [Google Scholar] [CrossRef]
- Villareal, D.T.; Murphy, W.A.; Teitelbaum, S.L.; Arens, M.Q.; Whyte, M.P. Painful Diffuse Osteosclerosis after Intravenous Drug Abuse. Am. J. Med. 1992, 93, 371–381. [Google Scholar] [CrossRef]
- Khosla, S.; Hassoun, A.A.K.; Baker, B.K.; Liu, F.; Zein, N.N.; Whyte, M.P.; Reasner, C.A.; Nippoldt, T.B.; Tiegs, R.D.; Hintz, R.L.; et al. Insulin-like Growth Factor System Abnormalities in Hepatitis C-Associated Osteosclerosis. Potential Insights into Increasing Bone Mass in Adults. J. Clin. Investig. 1998, 101, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, P.; Giuliani, N.; Fietta, P.; Mancini, C.; Lazzaretti, M.; Pollini, A.; Quaini, F.; Pedrazzoni, M. OPG/RANKL System Imbalance in a Case of Hepatitis C-Associated Osteosclerosis: The Pathogenetic Key? Clin. Rheumatol. 2005, 24, 296–300. [Google Scholar] [CrossRef]
- Fiore, C.E.; Riccobene, S.; Mangiafico, R.; Santoro, F.; Pennisi, P. Hepatitis C-Associated Osteosclerosis (HCAO): Report of a New Case with Involvement of the OPG/RANKL System. Osteoporos. Int. 2005, 16, 2180–2184. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Yang, S.H.; Brimble, M.A.; Callon, K.E.; Watson, M.; Park, Y.E.; Cornish, J. Synthesis of Truncated Analogues of Preptin-(1–16), and Investigation of Their Ability to Stimulate Osteoblast Proliferation. Bioorg. Med. Chem. 2014, 22, 3565–3572. [Google Scholar] [CrossRef] [PubMed]
- Amso, Z.; Kowalczyk, R.; Watson, M.; Park, Y.E.; Callon, K.E.; Musson, D.S.; Cornish, J.; Brimble, M.A. Structure Activity Relationship Study on the Peptide Hormone Preptin, a Novel Bone-Anabolic Agent for the Treatment of Osteoporosis. Org. Biomol. Chem. 2016, 14, 9225–9238. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Wang, J.; Yang, R.; Geller, D.S.; Loeb, D.M.; Hoang, B.H. Wnt Signaling in Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1258, 125–139. [Google Scholar] [CrossRef]
- Dreyer, T.J.; Keen, J.A.; Wells, L.M.; Roberts, S.J. Novel Insights on the Effect of Sclerostin on Bone and Other Organs. J. Endocrinol. 2023, 257, e220209. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, W.; Li, X.L. Effects of SOST Gene Silencing on Proliferation, Apoptosis, Invasion, and Migration of Human Osteosarcoma Cells through the Wnt/β-Catenin Signaling Pathway. Calcif. Tissue Int. 2017, 100, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Ideta, H.; Yoshida, K.; Okamoto, M.; Sasaki, J.; Kito, M.; Aoki, K.; Yoshimura, Y.; Suzuki, S.; Tanaka, A.; Takazawa, A.; et al. Antitumor Effect of Sclerostin against Osteosarcoma. Cancers 2021, 13, 6015. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Hookway, E.S.; Kashima, T.G.; Munemoto, M.; Tanaka, Y.; Hassan, A.B.; Oppermann, U.; Athanasou, N.A. Sclerostin Expression in Bone Tumours and Tumour-like Lesions. Histopathology 2016, 69, 470–478. [Google Scholar] [CrossRef]
- Märtson, A.; Kõks, S.; Reimann, E.; Prans, E.; Erm, T.; Maasalu, K. Transcriptome Analysis of Osteosarcoma Identifies Suppression of Wnt Pathway and Up-Regulation of Adiponectin as Potential Biomarker. Genom. Discov. 2013, 1, 3. [Google Scholar] [CrossRef]
- Reimann, E.; Kõks, S.; Ho, X.D.; Maasalu, K.; Märtson, A. Whole Exome Sequencing of a Single Osteosarcoma Case—Integrative Analysis with Whole Transcriptome RNA-Seq Data. Hum. Genom. 2014, 8, 20. [Google Scholar] [CrossRef]
- Rothzerg, E.; Ho, X.D.; Xu, J.; Wood, D.; Märtson, A.; Maasalu, K.; Kõks, S. Alternative Splicing of Leptin Receptor Overlapping Transcript in Osteosarcoma. Exp. Biol. Med. 2020, 245, 1437. [Google Scholar] [CrossRef] [PubMed]

| Research | Methods | Outcome |
|---|---|---|
| Xiao et al., 2019 [40] | Ob precursor cell line MC3T3-E1 | Preptin upregulates Wnt/β-catenin pathway, RUNX2 and osteocalcin |
| Cornish et al., 2007 [47] | Primary rat Ob-like cell line | Peptin administration:
|
| Adult male mice |
| |
| Liu et al., 2010 [52] | Human Ob |
|
| Bosetti et al., 2013 [62] | Human Ob | Preptin:
|
| Abdelfattah Abdulfadle K et al., 2023 [55] | Adult rats | Preptin:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, M.-C.; Bilha, S.C.; Hogas, M.; Velicescu, C.; Leustean, L.; Teodoriu, L.C.; Preda, C. Preptin: A New Bone Metabolic Parameter? Metabolites 2023, 13, 991. https://doi.org/10.3390/metabo13090991
Ungureanu M-C, Bilha SC, Hogas M, Velicescu C, Leustean L, Teodoriu LC, Preda C. Preptin: A New Bone Metabolic Parameter? Metabolites. 2023; 13(9):991. https://doi.org/10.3390/metabo13090991
Chicago/Turabian StyleUngureanu, Maria-Christina, Stefana Catalina Bilha, Mihai Hogas, Cristian Velicescu, Letitia Leustean, Laura Claudia Teodoriu, and Cristina Preda. 2023. "Preptin: A New Bone Metabolic Parameter?" Metabolites 13, no. 9: 991. https://doi.org/10.3390/metabo13090991