Impacts of Different Prenatal Supplementation Strategies on the Plasma Metabolome of Bulls in the Rearing and Finishing Phase
Abstract
:1. Introduction
2. Material and Methods
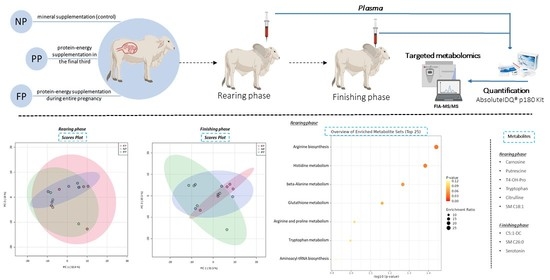
2.1. Experimental Design
2.2. Plasma Sample Collection and Processing
2.3. Targeted Metabolomics
2.4. Statistical Analysis
3. Results
3.1. Unsupervised Analysis of Metabolome (PCA)
3.2. Supervised Analysis of Metabolome (Multiple Linear Regression)
3.3. Repeated Measures Analysis over Time
3.4. Functional Enrichment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and Environmental Effects on Meat Quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.M.; Cromie, A.R.; Berry, D.P. Genetic Differences Based on a Beef Terminal Index Are Reflected in Future Phenotypic Performance Differences in Commercial Beef Cattle. Animal 2016, 10, 736–745. [Google Scholar] [CrossRef]
- Clavé, A.; Ripoll, G.; Casasús, I.; Sanz, A. Long-Term Effects of Early Maternal Undernutrition on the Growth, Physiological Profiles, Carcass and Meat Quality of Male Beef Offspring. Res. Vet. Sci. 2022, 142, 1–11. [Google Scholar] [CrossRef]
- Long, N.M.; Prado-Cooper, M.J.; Krehbiel, C.R.; Desilva, U.; Wettemann, R.P. Effects of Nutrient Restriction of Bovine Dams during Early Gestation on Postnatal Growth, Carcass and Organ Characteristics, and Gene Expression in Adipose Tissue and Muscle. J. Anim. Sci. 2010, 88, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Coloma-García, W.; Mehaba, N.; Llonch, P.; Caja, G.; Such, X.; Salama, A.A.K. Prenatal Heat Stress Effects on Gestation and Postnatal Behavior in Kid Goats. PLoS ONE 2020, 15, e0220221. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, C.R.; de Oliveira, B.F.; Gebim, P.G.H.; Édison, F.; Pontes, G.N.; Schmidt, P.D.A.; Guilherme, P.; de Almeida, S.M.H. Effects of Maternal Nutrition on Female Offspring Weight Gain and Sexual Development. Front. Genet. 2021, 12, 2059. [Google Scholar] [CrossRef]
- Schalch, F.J., Jr.; Polizel, G.H.G.; Cançado, F.A.C.Q.; Fernandes, A.C.; Mortari, I.; Pires, P.R.L.; Fukumasu, H.; Santana, M.H.d.A.; Saran Netto, A. Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves. Metabolites 2022, 12, 347. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Cançado, F.A.C.Q.; Dias, E.F.F.; Fernandes, A.C.; Cracco, R.C.; Carmona, B.T.; Castellar, H.H.; Poleti, M.D.; de Almeida Santana, M.H. Effects of Different Prenatal Nutrition Strategies on the Liver Metabolome of Bulls and Its Correlation with Body and Liver Weight. Metabolites 2022, 12, 441. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Espigolan, R.; Fantinato-Neto, P.; de Francisco Strefezzi, R.; Rangel, R.B.; de Carli, C.; Fernandes, A.C.; Dias, E.F.F.; Cracco, R.C.; de Almeida Santana, M.H. Different Prenatal Supplementation Strategies and Its Impacts on Reproductive and Nutrigenetics Assessments of Bulls in Finishing Phase. Vet. Res. Commun. 2022, 1–15. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; de Francisco Strefezzi, R.; Cracco, R.C.; Fernandes, A.C.; Zuca, C.B.; Castellar, H.H.; Baldin, G.C.; de Almeida Santana, M.H. Effects of Different Maternal Nutrition Approaches on Weight Gain and on Adipose and Muscle Tissue Development of Young Bulls in the Rearing Phase. Trop. Anim. Health Prod. 2021, 53, 1–9. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Fantinato-Neto, P.; Rangel, R.B.; Grigoletto, L.; de Oliveira Bussiman, F.; Cracco, R.C.; Garcia, N.P.; Ruy, I.M.; Ferraz, J.B.S.; de Almeida Santana, M.H. Evaluation of Reproductive Traits and the Effect of Nutrigenetics on Bulls Submitted to Fetal Programming. Livest. Sci. 2021, 247, 104487. [Google Scholar] [CrossRef]
- Elolimy, A.; Alharthi, A.; Zeineldin, M.; Parys, C.; Helmbrecht, A.; Loor, J.J. Supply of Methionine During Late-Pregnancy Alters Fecal Microbiota and Metabolome in Neonatal Dairy Calves Without Changes in Daily Feed Intake. Front. Microbiol. 2019, 10, 2159. [Google Scholar] [CrossRef]
- Block, J.J.; Webb, M.J.; Underwood, K.R.; Gonda, M.G.; Harty, A.A.; Salverson, R.R.; Funston, R.N.; Olson, K.C.; Blair, A.D. Influence of Maternal Protein Restriction in Primiparous Beef Heifers during Mid-and/or Late-Gestation on Progeny Feedlot Performance and Carcass Characteristics. Animals 2022, 12, 588. [Google Scholar] [CrossRef]
- McCoski, S.; Bradbery, A.; Marques, R.d.S.; Posbergh, C.; Sanford, C. Maternal Nutrition and Developmental Programming of Male Progeny. Animals 2021, 11, 2216. [Google Scholar] [CrossRef]
- Copping, K.J.; Hoare, A.; Callaghan, M.; McMillen, I.C.; Rodgers, R.J.; Perry, V.E.A.; Copping, K.J.; Hoare, A.; Callaghan, M.; McMillen, I.C.; et al. Fetal Programming in 2-Year-Old Calving Heifers: Peri-Conception and First Trimester Protein Restriction Alters Fetal Growth in a Gender-Specific Manner. Anim. Prod. Sci. 2014, 54, 1333–1337. [Google Scholar] [CrossRef]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Testa, L.M.; Long, N.M.; Quintans, G.I.; Pavan, E. The Influence of Protein Restriction during Mid- to Late Gestation on Beef Offspring Growth, Carcass Characteristic and Meat Quality. Meat Sci. 2019, 153, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Novais, F.J.; Pires, P.R.L.; Alexandre, P.A.; Dromms, R.A.; Iglesias, A.H.; Ferraz, J.B.S.; Styczynski, M.P.W.; Fukumasu, H. Identification of a Metabolomic Signature Associated with Feed Efficiency in Beef Cattle. BMC Genom. 2019, 20, 8. [Google Scholar] [CrossRef]
- Antonelo; Gómez, J.F.M.; Cônsolo, N.R.B.; Beline, M.; Colnago, L.A.; Schilling, W.; Zhang, X.; Suman, S.P.; Gerrard, D.E.; Balieiro, J.C.C.; et al. Metabolites and Metabolic Pathways Correlated With Beef Tenderness. Meat Muscle Biol. 2020, 4, 19–20. [Google Scholar] [CrossRef]
- Keusch, G.T. What Do -omics Mean for the Science and Policy of the Nutritional Sciences? Am. J. Clin. Nutr. 2006, 83, 520S–522S. [Google Scholar] [CrossRef]
- Vlaanderen, J.; Moore, L.E.; Smith, M.T.; Lan, Q.; Zhang, L.; Skibola, C.F.; Rothman, N.; Vermeulen, R. Application of OMICS Technologies in Occupational and Environmental Health Research; Current Status and Projections. Occup. Environ. Med. 2010, 67, 136–143. [Google Scholar] [CrossRef]
- Kitano, H. Systems Biology: A Brief Overview. Science 2002, 295, 1662–1664. [Google Scholar] [CrossRef] [PubMed]
- Shi-Kai, Y.; Run-Hui, L.; Hui-Zi, J.; Xin-Ru, L.; Ji, Y.; Lei, S.; Zhang, W.-D. Omics in Pharmaceutical Research: Overview, Applications, Challenges, and Future Perspectives. Chin. J. Nat. Med. 2015, 13, 3–21. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Ashrafian, H.; Sounderajah, V.; Glen, R.; Ebbels, T.; Blaise, B.J.; Kalra, D.; Kultima, K.; Spjuth, O.; Tenori, L.; Salek, R.M.; et al. Metabolomics: The Stethoscope for the Twenty-First Century. Med. Princ. Pract. 2021, 30, 301–310. [Google Scholar] [CrossRef]
- Liebal, U.W.; Phan, A.N.T.; Sudhakar, M.; Raman, K.; Blank, L.M. Machine Learning Applications for Mass Spectrometry-Based Metabolomics. Metabolites 2020, 10, 243. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439861905. [Google Scholar]
- Hipkiss, A.R.; Gaunitz, F. Inhibition of Tumour Cell Growth by Carnosine: Some Possible Mechanisms. Amino Acids 2014, 46, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Barca, A.; Ippati, S.; Urso, E.; Vetrugno, C.; Storelli, C.; Maffia, M.; Romano, A.; Verri, T. Carnosine Modulates the Sp1-Slc31a1/Ctr1 Copper-Sensing System and Influences Copper Homeostasis in Murine CNS-Derived Cells. Am. J. Physiol. Cell Physiol. 2019, 316, C235–C245. [Google Scholar] [CrossRef]
- Oppermann, H.; Alvanos, A.; Seidel, C.; Meixensberger, J.; Gaunitz, F. Carnosine Influences Transcription via Epigenetic Regulation as Demonstrated by Enhanced Histone Acetylation of the Pyruvate Dehydrogenase Kinase 4 Promoter in Glioblastoma Cells. Amino Acids 2019, 51, 61–71. [Google Scholar] [CrossRef]
- Cônsolo, N.R.B.; Buarque, V.L.M.; Silva, J.; Poleti, M.D.; Barbosa, L.C.G.S.; Higuera-Padilla, A.; Gómez, J.F.M.; Colnago, L.A.; Gerrard, D.E.; Saran Netto, A.; et al. Muscle and Liver Metabolomic Signatures Associated with Residual Feed Intake in Nellore Cattle. Anim. Feed Sci. Technol. 2021, 271, 114757. [Google Scholar] [CrossRef]
- Cui, J.; Pottosin, I.; Lamade, E.; Tcherkez, G. What Is the Role of Putrescine Accumulated under Potassium Deficiency? Plant Cell Environ. 2020, 43, 1331–1347. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Pshenichnov, M.R.; Salakhetdinova, Y.; Nesterova, L.Y. The Role of Putrescine and Potassium Transport in the Regulation of DNA Topology during Escherichia Coti Adaptation to Heat Stress. Mikrobiologiya 1998, 67, 601–606. [Google Scholar]
- Robert Michaud, M.; Benoit, J.B.; Lopez-Martinez, G.; Elnitsky, M.A.; Lee, R.E.; Denlinger, D.L. Metabolomics Reveals Unique and Shared Metabolic Changes in Response to Heat Shock, Freezing and Desiccation in the Antarctic Midge, Belgica Antarctica. J. Insect Physiol. 2008, 54, 645–655. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics Profiling of Serum and Urine in Three Beef Cattle Breeds Revealed Different Levels of Tolerance to Heat Stress. J. Agric. Food Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Isherwood, C.M.; Van der Veen, D.R.; Johnston, J.D.; Skene, D.J. Twenty-Four-Hour Rhythmicity of Circulating Metabolites: Effect of Body Mass and Type 2 Diabetes. FASEB J. 2017, 31, 5557–5567. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Fensom, G.K.; Rinaldi, S.; Scalbert, A.; Appleby, P.N.; Achaintre, D.; Gicquiau, A.; Gunter, M.J.; Ferrari, P.; Kaaks, R.; et al. Pre-Diagnostic Metabolite Concentrations and Prostate Cancer Risk in 1077 Cases and 1077 Matched Controls in the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2017, 15, 122. [Google Scholar] [CrossRef]
- Ghaffari, M.H.; Sadri, H.; Schuh, K.; Dusel, G.; Frieten, D.; Koch, C.; Prehn, C.; Adamski, J.; Sauerwein, H. Biogenic Amines: Concentrations in Serum and Skeletal Muscle from Late Pregnancy until Early Lactation in Dairy Cows with High versus Normal Body Condition Score. J. Dairy Sci. 2019, 102, 6571–6586. [Google Scholar] [CrossRef]
- Humer, E.; Khol-Parisini, A.; Metzler-Zebeli, B.U.; Gruber, L.; Zebeli, Q. Alterations of the Lipid Metabolome in Dairy Cows Experiencing Excessive Lipolysis Early Postpartum. PLoS ONE 2016, 11, e0158633. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Fragkos, K.C.; Forbes, A. Citrulline as a Marker of Intestinal Function and Absorption in Clinical Settings: A Systematic Review and Meta-Analysis. UEG J. 2018, 6, 181–191. [Google Scholar] [CrossRef]
- Gilbreath, K.R.; Bazer, F.W.; Satterfield, M.C.; Wu, G. Amino Acid Nutrition and Reproductive Performance in Ruminants; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1285. [Google Scholar]
- Kott, M.L.; Pancini, S.; Speckhart, S.L.; Kimble, L.N.; White, R.R.; Stewart, J.L.; Johnson, S.E.; Ealy, A.D. Effects of Mid-Gestational l-Citrulline Supplementation to Twin-Bearing Ewes on Umbilical Blood Flow, Placental Development, and Lamb Production Traits. Transl. Anim. Sci. 2021, 5, txab102. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost All about Citrulline in Mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A New Major Signaling Molecule or Just Another Player in the Pharmaconutrition Game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef]
- Dougherty, D.M.; Marsh-Richard, D.M.; Mathias, C.W.; Hood, A.J.; Addicott, M.A.; Moeller, F.G.; Morgan, C.J.; Badawy, A.A.B. Comparison of 50- and 100-g L-Tryptophan Depletion and Loading Formulations for Altering 5-HT Synthesis: Pharmacokinetics, Side Effects, and Mood States. Psychopharmacology 2008, 198, 431–445. [Google Scholar] [CrossRef]
- Marsh, D.M.; Dougherty, D.M.; Moeller, F.G.; Swann, A.C.; Spiga, R. Laboratory-Measured Aggressive Behavior of Women: Acute Tryptophan Depletion and Augmentation. Neuropsychopharmacology 2002, 26, 660–671. [Google Scholar] [CrossRef]
- Rambali, B. The Contribution of Cocoa Additive to Cigarette Smoking Addiction; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 2002. [Google Scholar]
- Sainio, E.L.; Pulkki, K.; Young, S.N. L-Tryptophan: Biochemical, Nutritional and Pharmacological Aspects. Amin. Acids 1996, 10, 21–47. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and Indole Metabolism in Immune Regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A Review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Laporta, J.; Moore, S.A.E.; Peters, M.W.; Peters, T.L.; Hernandez, L.L. Short Communication: Circulating Serotonin (5-HT) Concentrations on Day 1 of Lactation as a Potential Predictor of Transition-Related Disorders. J. Dairy Sci. 2013, 96, 5146–5150. [Google Scholar] [CrossRef]
- Laporta, J.; Moore, S.A.E.; Weaver, S.R.; Cronick, C.M.; Olsen, M.; Prichard, A.P.; Schnell, B.P.; Crenshaw, T.D.; Peñagaricano, F.; Bruckmaier, R.M.; et al. Increasing Serotonin Concentrations Alter Calcium and Energy Metabolism in Dairy Cows. J. Endocrinol. 2015, 226, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.H.; Tecott, L.H. Serotonin and the Regulation of Mammalian Energy Balance. Front. Neurosci. 2013, 7, 36. [Google Scholar] [CrossRef]
- Watanabe, H.; Saito, R.; Nakano, T.; Takahashi, H.; Takahashi, Y.; Sumiyoshi, K.; Sato, K.; Chen, X.; Okada, N.; Iwasaki, S.; et al. Effect of Peripheral 5-HT on Glucose and Lipid Metabolism in Wether Sheep. PLoS ONE 2014, 9, e88058. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Guzik, A.C.; Van Der Meulen, J.; Dekker, R.; Kogut, J.; Kerr, B.J.; Southern, L.L. Effects of Supplemental L-Tryptophan on Serotonin, Cortisol, Intestinal Integrity, and Behavior in Weanling Piglets. J. Anim. Sci. 2006, 84, 963–971. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Özçelik, R.; Hernandez, L.L.; Bruckmaier, R.M. Short Communication: Supplementation of Colostrum and Milk with 5-Hydroxy-L-Tryptophan Affects Immune Factors but Not Growth Performance in Newborn Calves. J. Dairy Sci. 2018, 101, 794–800. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, W.; Song, W.H.; Sun, P.; Jia, Z.H. Effects of Tryptophan Supplementation on Cashmere Fiber Characteristics, Serum Tryptophan, and Related Hormone Concentrations in Cashmere Goats. Domest. Anim. Endocrinol. 2012, 43, 239–250. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Hernandez, L.L.; Weaver, S.; Bruckmaier, R.M. Increased Serum Serotonin Improves Parturient Calcium Homeostasis in Dairy Cows. J. Dairy Sci. 2017, 100, 1580–1587. [Google Scholar] [CrossRef]
- Valente, E.E.L.; Klotz, J.L.; Ahn, G.; Harmon, D.L. Pattern of Postruminal Administration of L-Tryptophan Affects Blood Serotonin in Cattle. Domest. Anim. Endocrinol. 2021, 74, 106574. [Google Scholar] [CrossRef]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Batchuluun, B.; Al Rijjal, D.; Prentice, K.J.; Eversley, J.A.; Burdett, E.; Mohan, H.; Bhattacharjee, A.; Gunderson, E.P.; Liu, Y.; Wheeler, M.B. Elevated Medium-Chain Acylcarnitines Are Associated With Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic β-Cell Dysfunction. Diabetes 2018, 67, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty Acid Oxidation and Carnitine Palmitoyltransferase I: Emerging Therapeutic Targets in Cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Xiang, L.; Wei, J.; Tian, X.Y.; Wang, B.; Chan, W.; Li, S.; Tang, Z.; Zhang, H.; Cheang, W.S.; Zhao, Q.; et al. Comprehensive Analysis of Acylcarnitine Species in Db/Db Mouse Using a Novel Method of High-Resolution Parallel Reaction Monitoring Reveals Widespread Metabolic Dysfunction Induced by Diabetes. Anal. Chem. 2017, 89, 10368–10375. [Google Scholar] [CrossRef]
- Seiler, S.E.; Koves, T.R.; Gooding, J.R.; Wong, K.E.; Stevens, R.D.; Ilkayeva, O.R.; Wittmann, A.H.; DeBalsi, K.L.; Davies, M.N.; Lindeboom, L.; et al. Carnitine Acetyltransferase Mitigates Metabolic Inertia and Muscle Fatigue during Exercise. Cell Metab. 2015, 22, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Casals, N.; Zammit, V.; Herrero, L.; Fadó, R.; Rodríguez-Rodríguez, R.; Serra, D. Carnitine Palmitoyltransferase 1C: From Cognition to Cancer. Prog. Lipid Res. 2016, 61, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Raffler, J.; Lu, W.; Lee, J.J.; Abbey, D.; Saleheen, D.; Rabinowitz, J.D.; Bennett, M.J.; Hand, N.J.; Brown, C.; et al. Fine Mapping and Functional Analysis Reveal a Role of SLC22A1 in Acylcarnitine Transport. Am. J. Hum. Genet. 2017, 101, 489–502. [Google Scholar] [CrossRef]
- Ladeira, M.M.; Schoonmaker, J.P.; Swanson, K.C.; Duckett, S.K.; Gionbelli, M.P.; Rodrigues, L.M.; Teixeira, P.D. Review: Nutrigenomics of Marbling and Fatty Acid Profile in Ruminant Meat. Animal 2018, 12, s282–s294. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Nguyen, O.C.; Malau-Aduli, A.E.O. Main Regulatory Factors of Marbling Level in Beef Cattle. Vet. Anim. Sci. 2021, 14, 100219. [Google Scholar] [CrossRef]
- Pastenkos, G.; Miller, J.L.; Pritchard, S.M.; Nicola, A.V. Role of Sphingomyelin in Alphaherpesvirus Entry. J. Virol. 2019, 93, e01547-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, P.L.; Steinhardt, R.A. Loss, Restoration, and Maintenance of Plasma Membrane Integrity. J. Cell Biol. 1997, 137, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Functions of Lipid Rafts in Biological Membranes. Annu. Rev. Cell Dev. Biol. 1998, 14, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, A.; Simons, K. The Differential Miscibility of Lipids as the Basis for the Formation of Functional Membrane Rafts. Biochim. Biophys. Acta Rev. Biomembr. 1998, 1376, 467–479. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered Levels of Serum Sphingomyelin and Ceramide Containing Distinct Acyl Chains in Young Obese Adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Stanford, K.; Chaves, A.V.; Evans, P.R.; de Souza Figueiredo, E.E.; Ribeiro, G. Nutrition, Feeding and Management of Beef Cattle in Intensive and Extensive Production Systems. In Animal Agriculture: Sustainability, Challenges and Innovations; Academic Press: Cambridge, MA, USA, 2020; pp. 75–98. ISBN 9780128170526. [Google Scholar]
- Summer, A.; Lora, I.; Formaggioni, P.; Gottardo, F. Impact of Heat Stress on Milk and Meat Production. Anim. Front. 2019, 9, 39–46. [Google Scholar] [CrossRef]




| Ingredients | Mineral Supplement | Protein-Energy Supplement |
|---|---|---|
| Corn (%) | 35 | 60 |
| Soybean meal (%) | - | 30 |
| Dicalcium phosphate (%) | 10 | - |
| Urea 45% (%) | - | 2.5 |
| Salt (%) | 30 | 5 |
| Minerthal 160 MD (%) * | 25 | 2.5 |
| Total digestible nutrients (%) | 26.76 | 67.55 |
| Crude protein (%) | 2.79 | 24.78 |
| Non-protein nitrogen (%) | - | 7.03 |
| Acid detergent fiber (%) | 1.25 | 4.76 |
| Neutral detergent fiber (%) | 4.29 | 11.24 |
| Fat (%) | 1.26 | 2.61 |
| Calcium (g/kg) | 74.11 | 6.2 |
| Phosphorus (g/kg) | 59.38 | 7.24 |
| Metabolites | NP | PP | FP | p Value |
|---|---|---|---|---|
| Carnosine | 23.69 ± 0.749 a | 18.68 ± 0.882 b | 19.44 ± 1.111 b | 0.008 |
| Putrescine | 0.128 ± 0.014 a | 0.076 ± 0.011 b | 0.090 ± 0.005 ab | 0.020 |
| t4-OH-Pro | 41.66 ± 2.435 a | 35.76 ± 1.985 ab | 33.76 ± 0.838 b | 0.032 |
| Tryptophan | 41.57 ± 3.867 a | 38.70 ± 2.632 ab | 31.26 ± 1.178 b | 0.037 |
| Citrulline | 48.51 ± 2.712 a | 52.72 ± 3.223 ab | 59.54 ± 1.657 b | 0.041 |
| SM C18:1 | 6.805 ± 2.072 ab | 1.817 ± 0.997 a | 9.108 ± 2.147 b | 0.047 |
| Metabolites | NP | PP | FP | p Value |
|---|---|---|---|---|
| C5:1-DC | 0.008 ± 0.006 a | 0.005 ± 0.005 a | 0.012 ± 0.009 b | 0.001 |
| SM C26:0 | 0.068 ± 0.032 a | 0.049 ± 0.006 a | 0.384 ± 0.090 b | 0.002 |
| Serotonin | 0.414 ± 0.047 a | 0.910 ± 0.176 b | 0.646 ± 0.080 ab | 0.026 |
| Metabolites | p Values | |
|---|---|---|
| Time | Interaction (Treatment × Time) | |
| Taurine | <0.001 | 0.593 |
| Carnosine | <0.001 | 0.933 |
| Histidine | <0.001 | 0.825 |
| Proline | <0.001 | 0.626 |
| Sarcosine | <0.001 | 0.969 |
| Glutamine | <0.001 | 0.406 |
| Tryptophan | <0.001 | 0.544 |
| Serine | <0.001 | 0.825 |
| Leucine | <0.001 | 0.368 |
| Ornithine | 0.001 | 0.145 |
| Asparagine | 0.001 | 0.968 |
| Met-SO | 0.001 | 0.175 |
| Arginine | 0.001 | 0.459 |
| Creatinine | 0.002 | 0.184 |
| Lysine | 0.002 | 0.474 |
| Methionine | 0.004 | 0.781 |
| ADMA | 0.009 | 0.963 |
| Glycine | 0.009 | 0.075 |
| Kynurenine | 0.015 | 0.268 |
| Citrulline | 0.023 | 0.071 |
| C9 | 0.026 | 0.820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polizel, G.H.G.; Fernandes, A.C.; Furlan, É.; Prati, B.C.T.; Ferraz, J.B.S.; Santana, M.H.d.A. Impacts of Different Prenatal Supplementation Strategies on the Plasma Metabolome of Bulls in the Rearing and Finishing Phase. Metabolites 2023, 13, 259. https://doi.org/10.3390/metabo13020259
Polizel GHG, Fernandes AC, Furlan É, Prati BCT, Ferraz JBS, Santana MHdA. Impacts of Different Prenatal Supplementation Strategies on the Plasma Metabolome of Bulls in the Rearing and Finishing Phase. Metabolites. 2023; 13(2):259. https://doi.org/10.3390/metabo13020259
Chicago/Turabian StylePolizel, Guilherme Henrique Gebim, Arícia Christofaro Fernandes, Édison Furlan, Barbara Carolina Teixeira Prati, José Bento Sterman Ferraz, and Miguel Henrique de Almeida Santana. 2023. "Impacts of Different Prenatal Supplementation Strategies on the Plasma Metabolome of Bulls in the Rearing and Finishing Phase" Metabolites 13, no. 2: 259. https://doi.org/10.3390/metabo13020259