Copper Electrowinning from Supercritical Leachate of Printed Circuit Boards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanical Processing
2.2. PCBs Characterization
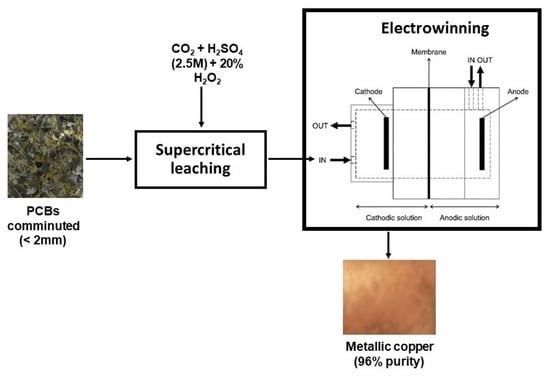
2.3. Supercritical Leaching
2.4. Copper Electrowinning
2.5. Experiments Using Synthetic Solution
2.6. Experiments Using Supercritical Leachate
3. Results and Discussion
3.1. PCBs Characterization
3.2. Supercritical Leachate
3.3. Copper Electrowinning
3.3.1. Copper Electrowinning from Synthetic Solutions
3.3.2. Purification of Supercritical Leachate
3.3.3. Copper Electrowinning from Supercritical Leachate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghosh, B.; Ghosh, M.; Parhi, P.; Mukherjee, P.; Mishra, B. Waste printed circuit boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Ning, C.; Lin, C.S.K.; Hui, D.C.W.; McKay, G. Waste printed circuit board (PCB) recycling techniques. Top. Curr. Chem. 2017, 375, 43. [Google Scholar] [CrossRef] [PubMed]
- Szałatkiewicz, J. Metals content in printed circuit board waste. Pol. J. Environ. Stud. 2014, 23, 2365–2369. [Google Scholar]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, B.; Pan, D.; Wu, Y.; Zuo, T. Recovery of waste printed circuit boards through pyrometallurgical processing: A review. Resour. Conserv. Recycl. 2017, 126, 209–218. [Google Scholar] [CrossRef]
- Guo, J.; Guo, J.; Xu, Z. Recycling of non-metallic fractions from waste printed circuit boards: A review. J. Hazard. Mater. 2009, 168, 567–590. [Google Scholar] [CrossRef]
- Marques, A.C.; Cabrera, J.-M.; de Fraga Malfatti, C. Printed circuit boards: A review on the perspective of sustainability. J. Environ. Manag. 2013, 131, 298–306. [Google Scholar] [CrossRef]
- Yamane, L.H.; Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Pickles, C.A. Potential and current practices of recycling waste printed circuit boards: A review of the recent progress in pyrometallurgy. J. Environ. Manag. 2022, 316, 115242. [Google Scholar] [CrossRef] [PubMed]
- Behnamfard, A.; Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Yang, J. Leaching copper from shredded particles of waste printed circuit boards. J. Hazard. Mater. 2011, 187, 393–400. [Google Scholar] [CrossRef]
- Preetam, A.; Jadhao, P.R.; Naik, S.N.; Pant, K.K.; Kumar, V. Supercritical fluid technology—An eco-freindly approach for resource recovery from e-waste and plastic waste—A review. Sep. Purif. Technol. 2023, 304, 12314. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Stopic, S.; Friedrich, B.; Tenório, J.A.S.; Espinosa, D.C.R. Cobalt Recovery from Li-Ion Battery Recycling: A critical Review. Metals. 2021, 11, 1999. [Google Scholar] [CrossRef]
- Reisdörfer, G.; Bertuol, D.A.; Tanabe, E.H. Extraction of neodymium from hard disk drives using supercritical CO2 with organic acids solutions acting as cosolvents. J. CO2 Util. 2020, 35, 277–287. [Google Scholar] [CrossRef]
- Argenta, A.B.; Reis, C.M.; Mello, G.P.; Dotto, G.L.; Tanabe, E.H.; Bertuol, D.A. Supercritical CO2 extraction of indium present in liquid crystal displays from discarded cell phones using organic acids. J. Supercrit. Fluids 2017, 120, 95–101. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Schlemmer, D.F.; da Silva, M.D.C.R.; Maziero, E.V.; Tanabe, E.H.; Bertuol, D.A. Fast copper extraction from printed circuit boards using supercritical carbon dioxide. Waste Manag. 2015, 45, 289–297. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Review: Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 16, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Liu, X.; Qu, G. Review: High value-added resource utilization of solid waste: Review of prospects for supercritical CO2 extraction of valuable metals. J. Clean. Prod. 2022, 372, 133813. [Google Scholar] [CrossRef]
- Mairizal, A.Q.; Sembada, A.Y.; Tse, K.M.; Rhamdhani, M.A. Electronic waste generation, economic values, distribution map, and possible recycling system in Indonesia. J. Clean. Prod. 2021, 293, 126096. [Google Scholar] [CrossRef]
- Sivakumar, P.; Prabhakaran, D.; Thirumarimurugan, M. Optimization Studies on Recovery of Metals from Printed Circuit Board Waste. Bioinorg. Chem. Appl. 2018, 2018, 1067512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajahalme, J.; Perämäki, S.; Budhathoki, R.; Väisänen, A. Effective Recovery Process of Copper from Waste Printed Circuit Boards Utilizing Recycling of Leachate. JOM 2021, 73, 980–987. [Google Scholar] [CrossRef]
- Baniasadi, M.; Graves, J.E.; Ray, D.A.; da Silva, A.L.; Renshaw, D.; Farnaud, S. Closed-Loop Recycling of Copper from Waste Printed Circuit Boards Using Bioleaching and Electrowinning Processes. Waste Biomass Valorization 2021, 12, 3125–3136. [Google Scholar] [CrossRef]
- Veit, H.M.; Bernardes, A.M.; Ferreira, J.Z.; Tenório, J.A.S.; Malffatti, C.F. Recovery of copper from printed circuit boards scraps by mechanical processing and electrometallurgy. J. Hazard. Mater. 2006, 137, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, Y.F.; Santos, I.D.; Dutra, A.J.B. Direct recovery of copper from printed circuit boards (PCBs) powder concentrate by a simultaneous electroleaching–electrodeposition process. Hydrometallurgy 2014, 149, 63–70. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Schlemmer, D.F.; Bassaco, M.M.; Dotto, G.L.; Tanabe, E.H.; Bertuol, D.A. Supercritical extraction of polymers from printed circuit boards using CO2 and ethanol. J. CO2 Util. 2017, 22, 307–316. [Google Scholar] [CrossRef]
- Hsu, E.; Durning, C.J.; West, A.C.; Park, A.A. Enhanced extraction of copper from electronic waste via induced morphological changes using supercritical CO2. Resour. Conserv. Recycl. 2021, 168, 105296. [Google Scholar] [CrossRef]
- Sanyal, S.; Ke, Q.; Zhang, Y.; Ngo, T.; Carrel, J.; Zhang, H.; Dai, L.L. Understanding and optimizing delamination/recycling of printed circuit boards using a supercritical carbon dioxide process. J. Clean. Prod. 2013, 41, 174–178. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, F.S. Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical water treatments. Chem. Eng. J. 2013, 219, 131–136. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liu, D.; Maiti, S.; Prempeh, N.; Hua, Y.; Lu, J. Recent Progress in Heavy Metal Extraction by Supercritical CO2 Fluids. Ind. Eng. Chem. Res. 2014, 53, 1866–1877. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Amado, F.D.R.; Veit, H.; Ferreira, J.Z.; Bernardes, A.M. Recovery of Nickel and Cobalt from Spent NiMH Batteries by Electrowinning. Chem. Eng. Technol. 2012, 35, 2084–2092. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 4th ed; Wiley Inter-Science: Hoboken, NJ, USA, 2000; pp. 13–87. [Google Scholar]
- HYDRA-MEDUSA. Chemical Equilibrium Diagrams. Available online: https://sites.google.com/site/chemdiagr/home?authuser=0 (accessed on 1 November 2022).
- Godirilwe, L.L.; Haga, K.; Altansukh, B.; Takasaki, Y.; Ishiyama, D.; Trifunovic, V.; Avramovic, L.; Jonovic, R.; Stevanovic, Z.; Shibayama, A. Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process. Metals 2021, 11, 1335. [Google Scholar] [CrossRef]
- Owais, A. Effect of electrolyte characteristics on electrowinning of copper powder. J. Appl. Electrochem. 2009, 39, 1587–1595. [Google Scholar] [CrossRef]
- Haghighi, H.K.; Moradkhani, D.; Sedaghat, B.; Najafabadi, M.R.; Behnamfard, A. Production of copper cathode from oxidized copper ores by acidic leaching and two-step precipitation followed by electrowinning. Hydrometallurgy 2013, 133, 111–117. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Imre-Lucaci, À.; Ilea, P. Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation. J. Hazard. Mater. 2014, 273, 215–221. [Google Scholar] [CrossRef]
- Kasper, A.C.; Berselli, G.B.T.; Freitas, B.D.; Tenório, J.A.S.; Bernardes, A.M.; Veit, H.M. Printed Wiring Boards for Mobile Phones: Characterization and Recycling of Copper. Waste Manag. 2011, 31, 2536–2545. [Google Scholar] [CrossRef]













| Element | Si | Fe | Cu | Zn | Ni | Impurities |
|---|---|---|---|---|---|---|
| Weight percentage (%) | 0.05 | 0.06 | 92.77 | 0.11 | 0.04 | 6.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calgaro, C.O.; da Silva, M.D.C.R.; Tanabe, E.H.; Bertuol, D.A. Copper Electrowinning from Supercritical Leachate of Printed Circuit Boards. Metals 2023, 13, 395. https://doi.org/10.3390/met13020395
Calgaro CO, da Silva MDCR, Tanabe EH, Bertuol DA. Copper Electrowinning from Supercritical Leachate of Printed Circuit Boards. Metals. 2023; 13(2):395. https://doi.org/10.3390/met13020395
Chicago/Turabian StyleCalgaro, Camila Ottonelli, Maurício Dalla Costa Rodrigues da Silva, Eduardo Hiromitsu Tanabe, and Daniel Assumpção Bertuol. 2023. "Copper Electrowinning from Supercritical Leachate of Printed Circuit Boards" Metals 13, no. 2: 395. https://doi.org/10.3390/met13020395