A Solid-State Pathway towards the Tunable Carboxylation of Cellulosic Fabrics: Controlling the Surface’s Acidity
Abstract
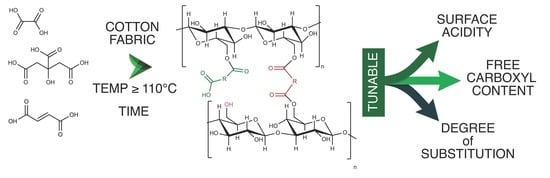
:1. Introduction
2. Materials and Methods
3. Results
3.1. Solid-State Carboxylation Procedure
3.2. Time of Reaction Experimental Optimization
3.3. Temperature of Reaction Experimental Optimization
3.4. FTIR
3.5. Surface Acidity
3.6. Dye Adsorption Isotherms
3.7. Absorption Kinetics
4. Discussion
- FCC/TCC = 0 implies that there are no free carboxyl groups and that all of the carboxyl groups form ester bonds.
- 0 > FCC/TCC > 1/2 indicates that there are free carboxyl groups and that ester bonds form.
- FCC/TCC = 1/2 means that there are no ester bonds present, hence representing the maximum number of free carboxyl groups at a given degree of substitution.
- FCC/TCC = 0 implies that there are no free carboxyl groups as they all form ester bonds.
- If 0 > FCC/TCC ≥ 1/3, there is an average of up to one COOH free.
- If 1/3 > FCC/TCC ≥ 2/3, there is an average of up to two COOH free.
- If FCC/TCC = 2/3, there is no crosslinking and the maximum amount of free carboxyl groups is achieved.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, T.; Isogai, A. Ion-Exchange Behavior of Carboxylate Groups in Fibrous Cellulose Oxidized by the TEMPO-Mediated System. Carbohydr. Polym. 2005, 61, 183–190. [Google Scholar] [CrossRef]
- da Silva Pinto, M.; Sierra-Avila, C.A.; Hinestroza, J.P. In Situ Synthesis of a Cu-BTC Metal–Organic Framework (MOF 199) onto Cellulosic Fibrous Substrates: Cotton. Cellulose 2012, 19, 1771–1779. [Google Scholar] [CrossRef]
- Kim, M.L.; Otal, E.H.; Hinestroza, J.P. Cellulose Meets Reticular Chemistry: Interactions between Cellulosic Substrates and Metal–Organic Frameworks. Cellulose 2019, 26, 123–137. [Google Scholar] [CrossRef]
- Schelling, M.; Kim, M.; Otal, E.; Aguirre, M.; Hinestroza, J.P. Synthesis of a Zinc–Imidazole Metal–Organic Framework (ZIF-8) Using ZnO Rods Grown on Cotton Fabrics as Precursors: Arsenate Absorption Studies. Cellulose 2020, 27, 6399–6410. [Google Scholar] [CrossRef]
- Schelling, M.; Kim, M.; Otal, E.; Hinestroza, J. Decoration of Cotton Fibers with a Water-Stable Metal–Organic Framework (UiO-66) for the Decomposition and Enhanced Adsorption of Micropollutants in Water. Bioengineering 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Huang, X.; Lin, W.; Chen, Y.; Lang, X.; Wang, Y.; Gao, L.; Zhu, H.; Chen, J. Selective Adsorption of Cationic Dyes for Stable Metal–Organic Framework ZJU-48. ACS Omega 2020, 5, 13595–13600. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal–Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Schramm, C.; Vukušić, S.B.; Katović, D. Non-Formaldehyde Durable Press Finishing of Dyed Fabrics: Evaluation of Cotton-Bound Polycarboxylic Acids. Coloration Technol. 2002, 118, 244–249. [Google Scholar] [CrossRef]
- Vukusic, S.B.; Katovic, D.; Schramm, C.; Trajkovic, J.; Sefc, B. Polycarboxylic Acids as Non-Formaldehyde Anti-Swelling Agents for Wood. Holzforschung 2006, 60, 439–444. [Google Scholar] [CrossRef]
- Wang, Z.; Hauser, P.J.; Laine, J.; Rojas, O.J. Multilayers of Low Charge Density Polyelectrolytes on Thin Films of Carboxymethylated and Cationic Cellulose. J. Adhes. Sci. Technol. 2011, 25, 643–660. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- One-Step Dispersion of Cellulose Nanofibers by Mechanochemical Esterification in an Organic Solvent—Huang—2012—ChemSusChem—Wiley Online Library. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/cssc.201200492 (accessed on 1 July 2021).
- Spinella, S.; Maiorana, A.; Qian, Q.; Dawson, N.J.; Hepworth, V.; McCallum, S.A.; Ganesh, M.; Singer, K.D.; Gross, R.A. Concurrent Cellulose Hydrolysis and Esterification to Prepare a Surface-Modified Cellulose Nanocrystal Decorated with Carboxylic Acid Moieties. ACS Sustain. Chem. Eng. 2016, 4, 1538–1550. [Google Scholar] [CrossRef]
- Sobkowicz, M.J.; Braun, B.; Dorgan, J.R. Decorating in Green: Surface Esterification of Carbon and Cellulosic Nanoparticles. Green Chem. 2009, 11, 680–682. [Google Scholar] [CrossRef]
- Da Silva Perez, D.; Montanari, S.; Vignon, M.R. TEMPO-Mediated Oxidation of Cellulose III. Biomacromolecules 2003, 4, 1417–1425. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A.; Aziz, N. Isotherms, Kinetics and Thermodynamics of Acid Dye Adsorption on Activated Palm Ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Sousa, H.R.; Silva, L.S.; Sousa, P.A.A.; Sousa, R.R.M.; Fonseca, M.G.; Osajima, J.A.; Silva-Filho, E.C. Evaluation of Methylene Blue Removal by Plasma Activated Palygorskites. J. Mater. Res. Technol. 2019, 8, 5432–5442. [Google Scholar] [CrossRef]
- Gürses, A.; Doğar, Ç.; Yalçın, M.; Açıkyıldız, M.; Bayrak, R.; Karaca, S. The Adsorption Kinetics of the Cationic Dye, Methylene Blue, onto Clay. J. Hazard. Mater. 2006, 131, 217–228. [Google Scholar] [CrossRef]
- Long, Y.; Yu, Y.; Chua, Y.W.; Wu, H. Acid-Catalysed Cellulose Pyrolysis at Low Temperatures. Fuel 2017, 193, 460–466. [Google Scholar] [CrossRef]
- Emsley, A.M.; Stevens, G.C. Kinetics and Mechanisms of the Low-Temperature Degradation of Cellulose. Cellulose 1994, 1, 26–56. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Dong, C. Levoglucosan Formation Mechanisms during Cellulose Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 104, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional Nanomaterials through Esterification of Cellulose: A Review of Chemistry and Application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Surface Acylation of Cellulose Whiskers by Drying Aqueous Emulsion. Biomacromolecules 2006, 7, 696–700. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, J.; Dong, X.; Ma, Y. Fiber Properties of Eucalyptus Kraft Pulp with Different Carboxyl Group Contents. Cellulose 2013, 20, 2839–2846. [Google Scholar] [CrossRef]
- Racz, I.; Borsa, J. Swelling of Carboxymethylated Cellulose Fibres. Cellulose 1997, 4, 293–303. [Google Scholar] [CrossRef]
- Li, D.; Henschen, J.; Ek, M. Esterification and Hydrolysis of Cellulose Using Oxalic Acid Dihydrate in a Solvent-Free Reaction Suitable for Preparation of Surface-Functionalised Cellulose Nanocrystals with High Yield. Green Chem. 2017, 19, 5564–5567. [Google Scholar] [CrossRef] [Green Version]
- Credou, J.; Berthelot, T. Cellulose: From Biocompatible to Bioactive Material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef] [Green Version]
- Berlioz, S.; Molina-Boisseau, S.; Nishiyama, Y.; Heux, L. Gas-Phase Surface Esterification of Cellulose Microfibrils and Whiskers. Biomacromolecules 2009, 10, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- New Approach for Single-Step Extraction of Carboxylated Cellulose Nanocrystals for Their Use As Adsorbents and Flocculants/ACS Sustainable Chemistry & Engineering. Available online: https://pubs.acs.org/doi/10.1021/acssuschemeng.6b00126 (accessed on 1 July 2021).
- Pantze, A.; Karlsson, O.; Westermark, U. Esterification of Carboxylic Acids on Cellulosic Material: Solid State Reactions. Holzforschung 2008, 62, 136–141. [Google Scholar] [CrossRef]
- Domínguez de María, P. Minimal Hydrolases: Organocatalytic Ring-Opening Polymerizations Catalyzed by Naturally Occurring Carboxylic Acids. ChemCatChem 2010, 2, 487–492. [Google Scholar] [CrossRef]
- Hafrén, J.; Córdova, A. Direct Organocatalytic Polymerization from Cellulose Fibers. Macromol. Rapid Commun. 2005, 26, 82–86. [Google Scholar] [CrossRef]
- Barret, R. 2—Importance and Evaluation of the pKa. In Therapeutical Chemistry; Barret, R., Ed.; Elsevier: Cham, Switzerland, 2018; pp. 21–51. ISBN 978-1-78548-288-5. [Google Scholar]
- Karaca, S.; Gürses, A.; Açıkyıldız, M.; Ejder (Korucu), M. Adsorption of Cationic Dye from Aqueous Solutions by Activated Carbon. Microporous Mesoporous Mater. 2008, 115, 376–382. [Google Scholar] [CrossRef]
- Bujdák, J.; Komadel, P. Interaction of Methylene Blue with Reduced Charge Montmorillonite. J. Phys. Chem. B 1997, 101, 9065–9068. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Montané, D.; Celzard, A. Adsorption of Phenol onto Activated Carbons Having Different Textural and Surface Properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]




| Optimal Time (min) | TCC (mmol/g) | FCC (mmol/g) | FCC/TCC | DS | |
|---|---|---|---|---|---|
| US—Oxalic | 90 | 4.0 | 1.1 | 0.29 | 0.58 |
| JP—Oxalic | 90 | 3.5 | 1.2 | 0.35 | 0.44 |
| US—Fumaric | 45 | 1.3 | 0.17 | 0.13 | 0.21 |
| JP—Fumaric | 45 | 1.5 | 0.22 | 0.15 | 0.27 |
| US—Citric | 60 | 2.2 | 0.35 | 0.16 | 0.45 |
| JP—Citric | 45 | 2.3 | 0.35 | 0.15 | 0.49 |
| Optimal Temperature (°C) | TCC (mmol/g) | FCC (mmol/g) | FCC/TCC | DS | |
|---|---|---|---|---|---|
| US—Fumaric | 110 | 1.3 | 0.17 | 0.13 | 0.20 |
| JP—Fumaric | 110 | 1.5 | 0.22 | 0.14 | 0.24 |
| US—Citric | 130 | 2.6 | 0.47 | 0.18 | 0.45 |
| JP—Citric | 130 | 2.7 | 0.55 | 0.20 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otal, E.H.; Kim, M.L.; Hinestroza, J.P.; Kimura, M. A Solid-State Pathway towards the Tunable Carboxylation of Cellulosic Fabrics: Controlling the Surface’s Acidity. Membranes 2021, 11, 514. https://doi.org/10.3390/membranes11070514
Otal EH, Kim ML, Hinestroza JP, Kimura M. A Solid-State Pathway towards the Tunable Carboxylation of Cellulosic Fabrics: Controlling the Surface’s Acidity. Membranes. 2021; 11(7):514. https://doi.org/10.3390/membranes11070514
Chicago/Turabian StyleOtal, Eugenio H., Manuela L. Kim, Juan P. Hinestroza, and Mutsumi Kimura. 2021. "A Solid-State Pathway towards the Tunable Carboxylation of Cellulosic Fabrics: Controlling the Surface’s Acidity" Membranes 11, no. 7: 514. https://doi.org/10.3390/membranes11070514