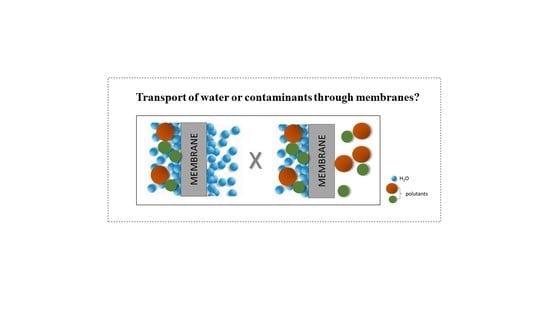
Membrane Removal of Emerging Contaminants from Water: Which Kind of Membranes Should We Use?
Abstract
:1. Introduction
- Membrane filtration; specially NF and RO due to very small pores (<2 nm).
- Granular and powdered activated carbon adsorption for rapid filters and seasonal uses.
- Advanced oxidation processes recommended as extra treatments especially for drinking water quality improvement.
- Graphene and graphene oxide adsorption due to their high and functionalized surface areas.
- Adsorption on carbon nanotubes with high adsorption performance and physicochemical properties (despite high costs).
- Biological degradation, the most used technique, requires space and time but is energy efficient.
- Metal organic framework adsorption (relatively new technology) [28].
2. Membranes for Water Treatment (WT)
3. Selectivity vs. Effectiveness of Membrane Separation
- Charge of the molecule
- Hydrophobicity of the separated molecule
- Size of the separated molecule
- Rejection of nonionic hydrophilic ECs (e.g., paracetamol, caffeine, methylparaben) is a result of physical sieving.
- Rejection of hydrophobic nonionic ECs (e.g., carbamazepine, estrone) is most influenced by the initial adsorption of the molecules on the membrane and, as the membrane gets saturated by the solute, the rejection decreases.
- Positively charged ECs (propranolol, metroprolol) and negatively charged (ibuprofen, naproxen, diclofenac) rejection is connected with the electrostatic interactions between the molecule and the membrane surface, and with sieving.
- Change of the surface charge (zeta potential).
- Change of the hydrophobicity of the membrane.
- Adsorption of some trace contaminant in the foulant.
- Change of the roughness of the membrane surface.
4. Dense Membrane Processes with Targeted Transport of ECs
- Note (a–c) Membrane separation based on pore size are the most employed membrane processes in WT such as UF, NF or RO. The processes are well described, membranes are available and the separation is fast. These processes were, however, not primarily designed for removal of ECs. There are several new studies trying to clarify whether UF, NF and RO may be successfully applied in removal of ECs. The rejection of different ECs by NF and RO were from 40 to 100% [59,60,61,74,77] depending on EC. Dense hydrophobic membranes for target transport of ECs (without the pore size mechanism) were not yet used.
- Note (d) The advantage of classical processes, with separation based on pore size, is that only one membrane type is needed for treatment of all contaminants, but insufficient selectivity would lead to additional separation parts. Using dense polymeric membranes for target transport of ECs, several different types would be needed.
- Note (e) Whether the molecule of EC is retained by the RO or NF membrane depends on the characteristics of the molecule, namely its charge, hydrophobicity and size. Which molecules will be retained, and which not, cannot be well predicted because it is not only a matter of sieving (as the method was originally designed). Example: “The molecular weight of the Hydroxybiphenyl is 170 g/mol and it should completely pass through the membrane’s pores if the size exclusion was the only mechanism of rejection. However, the rejection of Hydroxybiphenyl was nearly 100% because it was completely adsorbed on the membrane” [59]. Dense polymeric membranes are predictable for any kind of molecule.
- Note (f) After treatment by RO we get nearly UP water and brine full of different ECs compounds. Such water cannot be released to the environment or drunk [104]. It must be remineralized. No need when using targeted dense membranes because only the contaminants would be removed, and the natural composition of water will remain. In the case of water being contaminated mainly by one or two pollutants, it would be interesting to eliminate only these instead of the entire elimination of the water content (with uncertain results).
- Note (g) Membrane separation based on pore size suffers by fouling that can influence the interactions between the molecule and the membrane and, consequently, also the quality of rejection [81]; see also example in Note (e). The water passes along the dense polymeric membranes and, therefore, no fouling occurs.
4.1. Pervaporation
- (1)
- Membrane thickness.
- (2)
- Driving force.
- (3)
- Permeability of the membrane.
4.2. Pertraction
4.3. Membrane Extraction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| AOPs | advanced oxidation processes |
| CA | cellulose acetate |
| CNTs | carbon nanotubes |
| CS | chitosan |
| CTA | cellulose tri-acetate |
| DBCP | 1,2-dibromo-3-chloropropane |
| ECs | emerging contaminants |
| EDI | electrodeionization |
| FO | forward osmosis |
| GO | graphene oxide |
| HCH | hexachlorocyclohexane |
| LDPE | low-density polyethylene |
| MD | membrane distillation |
| MDg | membrane degasification |
| MF | microfiltration |
| MOFs | metal organic frameworks |
| NF | nanofiltration |
| PA | polyamide |
| PBI | polybenzimidazole |
| PDMS | polydimethyl siloxane |
| PEBA | polyether block amide |
| PEI | polyethyleneimine |
| PES | polyether sulfone |
| PET | polyethylene terephthalate |
| PU | polyurethane |
| PV | pervaporation |
| RO | reverse osmosis |
| SPEEK | sulfonated poly ether ether ketone |
| UF | ultrafiltration |
| WT | water treatment |
References
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.; Dickson, K.L.; Maki, A.W. Estimating the hazard of chemical substances to aquatic life. Hydrobiologia 1979, 64, 157–166. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Chander, V.; Sharma, B.; Negi, V.; Aswal, R.S.; Singh, P.; Singh, R.; Dobhal, R. Pharmaceutical compounds in drinking water. J. Xenobiotics 2016, 6, 5774. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An investigation of anticoccidial veterinary drugs as emerging organic contaminants in groundwater. Sci. Total Environ. 2020, 746, 141116. [Google Scholar] [CrossRef]
- Lin, X.; Xu, J.; Keller, A.A.; He, L.; Gu, Y.; Zheng, W.; Sun, D.; Lu, Z.; Huang, J.; Huang, X.; et al. Occurrence and risk assessment of emerging contaminants in a water reclamation and ecological reuse project. Sci. Total Environ. 2020, 744, 140977. [Google Scholar] [CrossRef]
- Mazille, F.; Schoettl, T.; Klamerth, N.; Malato, S.; Pulgarin, C. Field solar degradation of pesticides and emerging water contaminants mediated by polymer films containing titanium and iron oxide with synergistic heterogeneous photocatalytic activity at neutral pH. Water Res. 2010, 44, 3029–3038. [Google Scholar] [CrossRef]
- Matamoros, V.; Caiola, N.; Rosales, V.; Hernández, O.; Ibáñez, C. The role of rice fields and constructed wetlands as a source and a sink of pesticides and contaminants of emerging concern: Full-scale evaluation. Ecol. Eng. 2020, 156, 105971. [Google Scholar] [CrossRef]
- Badea, S.L.; Geana, E.I.; Niculescu, V.C.; Ionete, R.E. Recent progresses in analytical GC and LC mass spectrometric based-methods for the detection of emerging chlorinated and brominated contaminants and their transformation products in aquatic environment. Sci. Total Environ. 2020, 722, 137914. [Google Scholar] [CrossRef]
- Diuzheva, A.; Dejmkova, H.; Fischer, J.; Andruch, V. Simultaneous determination of three carbamate pesticides using vortex-assisted liquid-liquid microextraction combined with HPLC-amperometric detection. Microchem. J. 2019, 150, 104071. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Jung, C.; Son, A.; Her, N.; Zoh, K.D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Estrada, C.; Rostro-Alanis, M.D.; Munoz-Gutierrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldivar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation—A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemoller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Galindo-Miranda, J.M.; Guizar-Gonzalez, C.; Becerril-Bravo, E.J.; Moeller-Chavez, G.; Leon-Becerril, E.; Vallejo-Rodriguez, R. Occurrence of emerging contaminants in environmental surface waters and their analytical methodology—A review. Water Sci. Technol. Water Supply 2019, 19, 1871–1884. [Google Scholar] [CrossRef]
- Buser, H.R.; Poiger, T.; Muller, M.D. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Removal and seasonal variability of selected analgesics/anti-inflammatory, anti-hypertensive/cardiovascular pharmaceuticals and UV filters in wastewater treatment plant. Environ. Sci. Pollut. Res. 2014, 21, 7578–7585. [Google Scholar] [CrossRef] [PubMed]
- Rozman, D.; Hrkal, Z.; Váňa, M.; Vymazal, J.; Boukalová, Z. Occurrence of Pharmaceuticals in Wastewater and Their Interaction with Shallow Aquifers: A Case Study of Horní Beřkovice, Czech Republic. Water 2017, 9, 218. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ. Int. 2007, 33, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Herrmann, N.; Bonerz, M.; Knacker, T.; Siegrist, H.; Joss, A. A rapid method to measure the solid-water distribution coefficient (K-d) for pharmaceuticals and musk fragrances in sewage sludge. Water Res. 2004, 38, 4075–4084. [Google Scholar] [CrossRef]
- Vymazal, J.; Bfezinova, T. The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: A review. Environ. Int. 2015, 75, 11–20. [Google Scholar] [CrossRef]
- Vymazal, J.; Brezinova, T.; Kozeluh, M. Occurrence and removal of estrogens, progesterone and testosterone in three constructed wetlands treating municipal sewage in the Czech Republic. Sci. Total Environ. 2015, 536, 625–631. [Google Scholar] [CrossRef]
- Vymazal, J.; Brezinova, T.D.; Kozeluh, M.; Kule, L. Occurrence and removal of pharmaceuticals in four full-scale constructed wetlands in the Czech Republic—The first year of monitoring. Ecol. Eng. 2017, 98, 354–364. [Google Scholar] [CrossRef]
- Kumar, P.; Bansal, V.; Kim, K.H.; Kwon, E.E. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.Q.; Yang, W.B.; Yang, Z.; Li, X.M.; Zhou, X.; Liu, Y.; Shen, J.C.; Zhang, X.T. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Cukierman, A.L.; Nunell, G.V.; Bonelli, P.R. Removal of emerging pollutants from water through adsorption onto carbon-based materials. In Emerging and Nanomaterial Contaminants in Wastewater; Mishra, A.K., Anawar, H.M.D., Drouiche, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 7; pp. 159–213. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Nogales, R.; Romero, E. Innovative application of biobed bioremediation systems to remove emerging contaminants: Adsorption, degradation and bioaccesibility. Sci. Total Environ. 2019, 651, 990–997. [Google Scholar] [CrossRef]
- Gil, A.; Taoufik, N.; García, A.M.; Korili, S.A. Comparative removal of emerging contaminants from aqueous solution by adsorption on an activated carbon. Environ. Technol. 2019, 40, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Huang, J.-R. Adsorption of organic including pharmaceutical and inorganic contaminants in water toward graphene-based materials. In Contaminants of Emerging Concern in Water and Wastewater; Hernández-Maldonado, A.J., Blaney, L., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; Chapter 3; pp. 93–113. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.-W.; Jeon, B.-H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Yin, C.-Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Anguille, S.; Moulin, P.; Testa, F. Device for Extraction of Pollutants by Multichannel Tubular Membrane. U.S. Patent 10,137,385, 27 November 2018. [Google Scholar]
- Maoqi, F. Biochar Treatment of Contaminated Water. U.S. Patent 10,246,347, 2 April 2019. [Google Scholar]
- Nickelsen, M.G.; Woodard, S.E. Sustainable System and Method for Removing and Concentrating per-and Polyfluoroalkyl Substances (PFAS) from Water. U.S. Patent 10,287,185, 14 May 2019. [Google Scholar]
- Suri, R.P.; Bhattarai, B. Silica Particles Coated with β-Cyclodextrin for the Removal of Emerging Contaminants from Wastewater. U.S. Patent 9,828,458, 28 November 2017. [Google Scholar]
- Velazquez, F.R.; Alos, V.F.; Perez, O.P. Synthesis of Biocomposite Alginate-Chitosan-Magnetite Nanoparticle Beads for Removal of Organic Persistent Contaminants from Water Systems. U.S. Patent 10,569,253, 25 February 2020. [Google Scholar]
- Yu, M. Ultrathin, Graphene-Based Membranes for Water Treatment and Methods of their Formation and Use. U.S. Patent 10,092,882, 9 October 2018. [Google Scholar]
- Espindola, J.C.; Vilar, V.J.P. Innovative light -driven chemical/catalytic reactors towards contaminants of emerging concern mitigation: A review. Chem. Eng. J. 2020, 394. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Dolic, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- El Assal, Z.; Ojala, S.; Zbair, M.; Echchtouki, H.; Nevanpera, T.; Pitkaaho, S.; Pirault-Roy, L.; Bensitel, M.; Brahmi, R.; Keiski, R.L. Catalytic abatement of dichloromethane over transition metal oxide catalysts: Thermodynamic modelling and experimental studies. J. Clean. Prod. 2019, 228, 814–823. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Laitinen, T.; Pirila, M.; Keiski, R.L.; Ojala, S. Photocatalytic Degradation of Perfluorooctanoic Acid (PFOA) From Wastewaters by TiO2, In2O3 and Ga2O3 Catalysts. Top. Catal. 2017, 60, 1345–1358. [Google Scholar] [CrossRef]
- Sassi, H.; Lafaye, G.; Ben Amor, H.; Gannouni, A.; Jeday, M.R.; Barbier, J. Wastewater treatment by catalytic wet air oxidation process over Al-Fe pillared clays synthesized using microwave irradiation. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Espindola, J.C.; Cristovao, R.O.; Araujo, S.R.F.; Neuparth, T.; Santos, M.M.; Montes, R.; Quintana, J.B.; Rodil, R.; Boaventura, R.A.R.; Vilar, V.J.P. An innovative photoreactor, FluHelik, to promote UVC/H2O2 photochemical reactions: Tertiary treatment of an urban wastewater. Sci. Total Environ. 2019, 667, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; De Carluccio, M.; Eckert, E.M.; Fontaneto, D.; Fiorentino, A.; Corno, G.; Prete, P.; Cucciniello, R.; Proto, A.; Rizzo, L. Combination of flow cytometry and molecular analysis to monitor the effect of UVC/H2O2 vs UVC/H2O2/Cu-IDS processes on pathogens and antibiotic resistant genes in secondary wastewater effluents. Water Res. 2020, 184, 116194. [Google Scholar] [CrossRef] [PubMed]
- Diez, A.M.; Moreira, F.C.; Marinho, B.A.; Espindola, J.C.A.; Paulista, L.O.; Sanroman, M.A.; Pazos, M.; Boaventura, R.A.R.; Vilar, V.J.P. A step forward in heterogeneous photocatalysis: Process intensification by using a static mixer as catalyst support. Chem. Eng. J. 2018, 343, 597–606. [Google Scholar] [CrossRef]
- Stathoulopoulos, A.; Mantzavinos, D.; Frontistis, Z. Coupling Persulfate-Based AOPs: A Novel Approach for Piroxicam Degradation in Aqueous Matrices. Water 2020, 12, 1530. [Google Scholar] [CrossRef]
- Steinle-Darling, E.; Zedda, M.; Plumlee, M.H.; Ridgway, H.F.; Reinhard, M. Evaluating the impacts of membrane type, coating, fouling, chemical properties and water chemistry on reverse osmosis rejection of seven nitrosoalklyamines, including NDMA. Water Res. 2007, 41, 3959–3967. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Elimelech, M. Pharmaceutical retention mechanisms by nanofiltration membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef]
- Singh, R.; Hankins, N. Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Xie, Z.; Li, N.; Wang, Q.; Bolto, B. Desalination by pervaporation. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–226. [Google Scholar]
- Eyvaz, M.; Yüksel, E. Desalination and Water Treatment; IntechOpen: London, UK, 2018. [Google Scholar]
- Acero, J.L.; Benitez, F.J.; Teva, F.; Leal, A.I. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration. Chem. Eng. J. 2010, 163, 264–272. [Google Scholar] [CrossRef]
- Urtiaga, A.M.; Pérez, G.; Ibáñez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef]
- D’Haese, A.; Le-Clech, P.; Van Nevel, S.; Verbeken, K.; Cornelissen, E.R.; Khan, S.J.; Verliefde, A.R.D. Trace organic solutes in closed-loop forward osmosis applications: Influence of membrane fouling and modeling of solute build-up. Water Res. 2013, 47, 5232–5244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Albasi, C.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Dorandeu, C.; Marion, B.; Causserand, C. Cyclophosphamide removal from water by nanofiltration and reverse osmosis membrane. Water Res. 2009, 43, 4115–4122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of chitosan-based membranes for the separation of essential oil components by target-organophilic pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Z.; Liu, S.J.; Wang, J.; Li, Y.B.; Zhu, L.; Xu, M.; Wang, C.W. Hydrophilic SPEEK/PES composite membrane for pervaporation desalination. Sep. Purif. Technol. 2020, 250, 117265. [Google Scholar] [CrossRef]
- Cheng, X.X.; Pan, F.S.; Wang, M.R.; Li, W.D.; Song, Y.M.; Liu, G.H.; Yang, H.; Gao, B.X.; Wu, H.; Jiang, Z.Y. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Liu, G.P.; Wei, W.; Wu, H.; Dong, X.L.; Jiang, M.; Jin, W.Q. Pervaporation performance of PDMS/ceramic composite membrane in acetone butanol ethanol (ABE) fermentation-PV coupled process. J. Membr. Sci. 2011, 373, 121–129. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, X.; Tian, G.; Xuehong, G.; Liu, Z. Pervaporation of phenol wastewater with PEBA–PU blend membrane. Desalin. Water Treat. 2018, 102, 101–109. [Google Scholar] [CrossRef]
- Halakoo, E.; Feng, X. Layer-by-layer assembly of polyethyleneimine/graphene oxide membranes for desalination of high-salinity water via pervaporation. Sep. Purif. Technol. 2020, 234, 116077. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. 1-Fundamentals of pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–17. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Luis, P. Chapter Four—Pervaporation. In Progress in Filtration and Separation; Tarleton, S., Ed.; Academic Press: Oxford, UK, 2015; pp. 101–154. [Google Scholar] [CrossRef]
- Dhangar, K.; Kumar, M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: A review. Sci. Total Environ. 2020, 738, 140320. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, B.; Schaep, J.; Wilms, D.; Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Maeng, S.K.; Fujioka, T.; Kennedy, M.; Li, Z.Y.; Amy, G. Nanofiltration vs. reverse osmosis for the removal of emerging organic contaminants in water reuse. Desalin. Water Treat. 2011, 34, 50–56. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Elimelech, M. Role of electrostatic interactions in the retention of pharmaceutically active contaminants by a loose nanofiltration membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Childress, A.E.; Elimelech, M. Relating nanofiltration membrane performance to membrane charge (electrokinetic) characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Maeng, S.K.; Fujioka, T.; Kennedy, M.; Amy, G. Proposing nanofiltration as acceptable barrier for organic contaminants in water reuse. J. Membr. Sci. 2010, 362, 334–345. [Google Scholar] [CrossRef]
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Jang, A. Effect of forward osmosis (membrane) support layer fouling by organic matter in synthetic seawater solution. Desalin. Water Treat. 2016, 57, 24595–24605. [Google Scholar] [CrossRef]
- Linares, R.V.; Yangali-Quintanilla, V.; Li, Z.Y.; Amy, G. Rejection of micropollutants by clean and fouled forward osmosis membrane. Water Res. 2011, 45, 6737–6744. [Google Scholar] [CrossRef]
- Agenson, K.O.; Urase, T. Change in membrane performance due to organic fouling in nanofiltration (NF)/reverse osmosis (RO) applications. Sep. Purif. Technol. 2007, 55, 147–156. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel technologies for reverse osmosis concentrate treatment: A review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef]
- Kaminski, W.; Marszalek, J.; Tomczak, E. Water desalination by pervaporation–Comparison of energy consumption. Desalination 2018, 433, 89–93. [Google Scholar] [CrossRef]
- Araki, S.; Gondo, D.; Yamamoto, H. Pervaporation properties and a semi-empirical model for removal of VOCs from water using a propyl functionalized silica membrane. Desalin. Water Treat. 2019, 143, 17–23. [Google Scholar] [CrossRef]
- Higuchi, A.; Yoon, B.O.; Asano, T.; Nakaegawa, K.; Miki, S.; Hara, M.; He, Z.J.; Pinnau, I. Separation of endocrine disruptors from aqueous solutions by pervaporation. J. Membr. Sci. 2002, 198, 311–320. [Google Scholar] [CrossRef]
- Higuchi, A.; Yoon, B.O.; Kaneko, T.; Ham, M.; Maekawa, M.; Nohmi, T. Separation of endocrine disruptors from aqueous solutions by pervaporation: Dioctylphthalate and butylated hydroxytoluene in mineral water. J. Appl. Polym. Sci. 2004, 94, 1737–1742. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Bolto, B.; Hoang, M.; Xie, Z. Desalination by pervaporation: A review. Desalination 2016, 387, 46–60. [Google Scholar] [CrossRef]
- Egea-Corbacho, A.; Gutierrez, S.; Quiroga, J.M. Removal of emerging contaminants from wastewater through pilot plants using intermittent sand/coke filters for its subsequent reuse. Sci. Total Environ. 2019, 646, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ye, Y.Y.; Ma, H.M.; Zhang, Y.; Guo, W.S.; Du, B.; Wei, Q.; Wei, D.; Ngo, H.H. A critical review on membrane hybrid system for nutrient recovery from wastewater. Chem. Eng. J. 2018, 348, 143–156. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E. The role of membrane surface charge and solute physico-chemical properties in the rejection of organic acids by NF membranes. J. Membr. Sci. 2005, 249, 227–234. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Kim, T.U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Coleman, P.J.; Espendiller, C. Mechanisms underlying the effects of membrane fouling on the nanofiltration of trace organic contaminants. Desalination 2010, 250, 682–687. [Google Scholar] [CrossRef]
- Bellona, C.; Marts, M.; Drewes, J.E. The effect of organic membrane fouling on the properties and rejection characteristics of nanofiltration membranes. Sep. Purif. Technol. 2010, 74, 44–54. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Elimelech, M. Removal of natural hormones by nanofiltration membranes: Measurement, modeling, and mechanisms. Environ. Sci. Technol. 2004, 38, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Anumol, T.; Roccaro, P.; Vagliasindi, F.G.A.; Snyder, S.A. Modeling emerging contaminants breakthrough in packed bed adsorption columns by UV absorbance and fluorescing components of dissolved organic matter. Water Res. 2018, 145, 667–677. [Google Scholar] [CrossRef]
- Vergili, I. Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J. Environ. Manag. 2013, 127, 177–187. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid Ultrafiltration/Photocatalytic Membranes for Efficient Water Treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392, 192–203. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211, 304–316. [Google Scholar] [CrossRef]
- Lin, J.C.T.; Lee, D.J.; Huang, C.P. Membrane Fouling Mitigation: Membrane Cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2016, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. Environmental impact assessment of advanced urban wastewater treatment technologies for the removal of priority substances and contaminants of emerging concern: A review. J. Clean. Prod. 2020, 261, 1210178. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.M.; Eckelman, M.J.; Onnis-Hayden, A.; Gu, A.Z. Comparative Life Cycle Assessment of Advanced Wastewater Treatment Processes for Removal of Chemicals of Emerging Concern. Environ. Sci. Technol. 2018, 52, 11346–11358. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.X.; Zhang, W.L.; Xiong, W.; Ye, Q.L.; Hou, X.; Wang, C.; Wang, P.F. Life cycle assessment of advanced wastewater treatment processes: Involving 126 pharmaceuticals and personal care products in life cycle inventory. J. Environ. Manag. 2019, 238, 442–450. [Google Scholar] [CrossRef]
- Castro-Munoz, R. Pervaporation: The emerging technique for extracting aroma compounds from food systems. J. Food Eng. 2019, 253, 27–39. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 927068. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.P.; Dai, Y.; Xu, Y.; Wu, Y.M.; Cui, P.Z.; Zhu, Z.Y.; Ma, Y.X.; Wang, Y.L. Energy, economic and environmental evaluations for the separation of ethyl acetate/ethanol/water mixture via distillation and pervaporation unit. Process Saf. Environ. Prot. 2020, 140, 14–25. [Google Scholar] [CrossRef]
- Mei, X.; Ding, Y.; Li, P.P.; Xu, L.J.; Wang, Y.; Guo, Z.W.; Shen, W.T.; Yang, Y.; Wang, Y.H.; Xiao, Y.Y.; et al. A novel system for zero-discharge treatment of high-salinity acetonitrile-containing wastewater: Combination of pervaporation with a membrane-aerated bioreactor. Chem. Eng. J. 2020, 384, 123338. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, X.; Ma, T.F.; Xue, C.J.; Wu, M.D.; Ji, M.; Li, Y.G. Green recovery of hazardous acetonitrile from high-salt chemical wastewater by pervaporation. J. Clean. Prod. 2018, 197, 742–749. [Google Scholar] [CrossRef]
- Tgarguifa, A.; Abderafi, S.; Bounahmidi, T. Energy efficiency improvement of a bioethanol distillery, by replacing a rectifying column with a pervaporation unit. Renew. Energy 2018, 122, 239–250. [Google Scholar] [CrossRef]
- Lipski, C.; Cote, P. The Use of Pervaporation for the Removal of Organic Contaminants from Water. Environ. Prog. 1990, 9, 254–261. [Google Scholar] [CrossRef]
- Feng, X.S.; Huang, R.Y.M. Liquid separation by membrane pervaporation: A review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, Permeance and Selectivity: A preferred Way of Reporting Pervaporation Performance Data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Gani, K.M.; Kazmi, A.A. Comparative assessment of phthalate removal and risk in biological wastewater treatment systems of developing countries and small communities. Sci. Total Environ. 2016, 569, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Reynolds, T.; White, R.; Parker, M.G.; Sumpter, J.P. A Variety of Environmentally Persistent Chemicals, Including Some Phthalate Plasticizers, Are Weakly Estrogenic. Environ. Health Perspect. 1995, 103, 582–587. [Google Scholar] [CrossRef]
- Yoon, B.O.; Koyanagi, S.; Asano, T.; Hara, M.; Higuchi, A. Removal of endocrine disruptors by selective sorption method using polydimethylsiloxane membranes. J. Membr. Sci. 2003, 213, 137–144. [Google Scholar] [CrossRef]
- Waters, L.J.; Bhuiyan, A.K.M.M.H. Ionisation effects on the permeation of pharmaceutical compounds through silicone membrane. Colloids Surf. B Biointerfaces 2016, 141, 553–557. [Google Scholar] [CrossRef]
- Bhuiyan, A.K.M.M.H.; Waters, L.J. Permeation of pharmaceutical compounds through silicone membrane in the presence of surfactants. Colloid Surf. A 2017, 516, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Sauve, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, E.R.; Chemburk, P.B. Evaluation Control and Prediction of Drug Diffusion through Polymeric Membranes I. Methods Reproducibility of Steady-State Diffusion Studies. J. Pharm. Sci. 1968, 57, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R.; Chemburk, P.B. Evaluation Control and Prediction of Drug Diffusion through Polymeric Membranes II. Diffusion of Aminophenones through Silastic Membranes—A Test of Ph-Partition Hypothesis. J. Pharm. Sci. 1968, 57, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R.; Chemburkar, P.B. Evaluation Control and Prediction of Drug Diffusion through Polymeric Membranes III. Diffusion of Barbiturates Phenylalkylamines Dextromethorphan Progesterone and Other Drugs. J. Pharm. Sci. 1968, 57, 1401–1409. [Google Scholar] [CrossRef]
- Brouwer, E.R.; Lingeman, H.; Brinkman, U.A.T. Use of membrane extraction disks for on-line trace enrichment of organic compounds from aqueous samples. Chromatographia 1990, 29, 415–418. [Google Scholar] [CrossRef]
- Poliwoda, A.; Krzyżak, M.; Wieczorek, P.P. Supported liquid membrane extraction with single hollow fiber for the analysis of fluoroquinolones from environmental surface water samples. J. Chromatogr. A 2010, 1217, 3590–3597. [Google Scholar] [CrossRef]
- Megersa, N.; Chimuka, L.; Solomon, T.; Jönsson, J.Å. Automated liquid membrane extraction and trace enrichment of triazine herbicides and their metabolites in environmental and biological samples. J. Sep. Sci. 2001, 24, 567–576. [Google Scholar] [CrossRef]
- Guo, X.; Mitra, S. Development of pulse introduction membrane extraction for analysis of volatile organic compounds in individual aqueous samples, and for continuous on-line monitoring. J. Chromatogr. A 1998, 826, 39–47. [Google Scholar] [CrossRef]
- Prieto, A.; Telleria, O.; Etxebarria, N.; Fernández, L.A.; Usobiaga, A.; Zuloaga, O. Simultaneous preconcentration of a wide variety of organic pollutants in water samples: Comparison of stir bar sorptive extraction and membrane-assisted solvent extraction. J. Chromatogr. A 2008, 1214, 1–10. [Google Scholar] [CrossRef]
- Ray, S.K.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Perstraction of Phenolic Compounds from Aqueous Solution Using a Nonporous Membrane. Sep. Sci. Technol. 1997, 32, 2669–2683. [Google Scholar] [CrossRef]
- Rodil, R.; Schrader, S.; Moeder, M. Non-porous membrane-assisted liquid–liquid extraction of UV filter compounds from water samples. J. Chromatogr. A 2009, 1216, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Schrader, S.; Moeder, M. Pressurised membrane-assisted liquid extraction of UV filters from sludge. J. Chromatogr. A 2009, 1216, 8851–8858. [Google Scholar] [CrossRef] [PubMed]
- Villaverde-de-Sáa, E.; González-Mariño, I.; Quintana, J.B.; Rodil, R.; Rodríguez, I.; Cela, R. In-sample acetylation-non-porous membrane-assisted liquid–liquid extraction for the determination of parabens and triclosan in water samples. Anal. Bioanal. Chem. 2010, 397, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Yamini, Y.; Reimann, C.T.; Vatanara, A.; Jönsson, J.Å. Extraction and preconcentration of salbutamol and terbutaline from aqueous samples using hollow fiber supported liquid membrane containing anionic carrier. J. Chromatogr. A 2006, 1124, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Einsle, T.; Paschke, H.; Bruns, K.; Schrader, S.; Popp, P.; Moeder, M. Membrane-assisted liquid–liquid extraction coupled with gas chromatography–mass spectrometry for determination of selected polycyclic musk compounds and drugs in water samples. J. Chromatogr. A 2006, 1124, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Hylton, K.; Mitra, S. Automated, on-line membrane extraction. J. Chromatogr. A 2007, 1152, 199–214. [Google Scholar] [CrossRef]
- Jönsson, J.Å.; Mathiasson, L. Membrane-based techniques for sample enrichment. J. Chromatogr. A 2000, 902, 205–225. [Google Scholar] [CrossRef]
| Category | Name | M (g/mol) | Log Kow | MP (°C) | p sat at 25 °C (Pa) | w solub (mg/L) |
|---|---|---|---|---|---|---|
| Hormones | 117-b-estradiol | 272.4 | 4.01 | 176 | 8.50 × 10−7 | 3.6 |
| Hormones | Gestodene | 270.4 | 3.13 | 255 | 30 | |
| Antibiotics | Sulfamethoxazole | 253.3 | 0.89 | 167 | 9.23 × 10−6 | 610 |
| Antibiotics | Sulfapyridine | 250.3 | 0.35 | 192 | 268 | |
| Lipid regulators | Clofibrate | 242.7 | 3.02 | <25 | insoluble | |
| Lipid regulators | Gemifibrozil | 250.3 | 4.77 | 62 | 4.10 × 10−3 | 11 |
| NAIDs | Ibuprofen | 206.3 | 3.97 | 76 | 6.30 × 10−3 | 21 |
| NAIDs | Aspirin | 180.2 | 1.19 | 135 | 3.60 × 10−3 | 4600 |
| NAIDs | Diclofenac | 296.1 | 4.51 | 285 | 8.82 × 10−5 | 2.37 |
| NAIDs | Paracetamol | 151.2 | 0.33 | 170 | 8.40 × 10−3 | 14,000 |
| Betablockers | Pindolol | 248.3 | 1.75 | 170 | 7880 | |
| Betablockers | Propranolol | 259.3 | 3.48 | 96 | 61 | |
| Anti-depressants | Amitriptiline | 277.4 | 4.81 | 196 | 4.80 × 10−5 | 9.71 |
| Anti-depressants | Meprobamate | 218.3 | 0.93 | 105 | 4.00 × 10−1 | 4700 |
| Anti-convulsants | Carbamazepine | 236.3 | 13.9 | 190 | 2.40 × 10−5 | 17.7 |
| Anti-convulsants | Cabapentine | 171.2 | −1.10 | 165 | 4.00 × 10−8 | 4490 |
| Preservatives | 2-phenoxyethanol | 138.2 | 1.16 | 14 | 1.30 × 100 | 24,000 |
| Preservatives | Methylparaben | 151.2 | 1.96 | 131 | 3.20 × 10−2 | 2500 |
| Preservatives | Ethyl-4-hydroxy benzoate | 166.2 | 2.47 | 117 | 1.20 × 10−2 | 885 |
| Disinfectants | 2-phenylphenol | 170.2 | 3.09 | 60 | 2.70 × 10−1 | 700 |
| Disinfectants | Chloroprene | 88.5 | 2.2 | −130 | 2.51 × 104 | 260 |
| Disinfectants | Bromoprene | 133 | −126 | 7.35 × 103 | ||
| Plasticizers | Diethyl phthalate | 222.2 | 2.47 | −4 | 2.60 × 10−1 | 1080 |
| Plasticizers | Di(2-ethylhexyl) phthalate | 390.6 | 7.6 | −55 | 1.80 × 10−5 | 0.27 |
| Plasticizers | Benzylbutyl phthalate | 312.3 | 4.73 | −35 | 1.10 × 10−3 | 2.69 |
| Plasticizers | Bis(2-ethylhexyl) adipate | 370.6 | 8.1 | −70 | 1.13 × 10−4 | 0.1 |
| Plasticizers | Dibutylphthalate | 278.3 | 4.5 | −35 | 2.70 × 10−3 | 11.2 |
| Plasticizers | 1,2-dibromo-3-chloropropane | 236.6 | 2.96 | 6 | 1.07 × 102 | 1.23 |
| Pesticides | Glyphosate | 168.1 | −3.20 | 189 | 1.50 × 10−5 | 1200 |
| Process | Nominal Pore Size | Driving Force | Membrane Type | Use |
|---|---|---|---|---|
| Microfiltration (MF) | 0.05–10 µm | Transmembrane pressure diff. 1–3 bar | Porous, as/symmetric | Filtration |
| Ultrafiltration (UF) | 0.001–0.005 µm | Transmembrane pressure diff. 2–5 bar | Microporous asymmetric (PES, TF, CA) * | Filtr. protein and pathogen [59,60] |
| Nanofiltration (NF) | <2 nm | Transmembrane pressure diff. 5–15 bar | Thin film comp., porous (PA, PS) * | Filtration-large ions [61] |
| Forward Osmosis (FO) | 0.5 nm | Osmotic pressure | Asymm., thin film composite (CTA) * | Desalination [62] |
| Reverse Osmosis (RO) | 0.5 nm | Transmembrane pressure diff. 15–75 bar | Asymm., thin film composite (PA, PBI) * | Desalination [60,61,63] |
| Electrodialysis (ED) | MW < 200 Da | Electrical potential | Swollen gel, charged, symm. | Desalination |
| Electrodeionization (EDI) | MW < 200 Da | Electrical potential | Swollen gel, charged, symm. | Desalination |
| Membrane degasification (MDg) | 0.05–0.1 µm | Transmembrane pressure diff., vacuum on perm. side | Porous, symmetric or asymmetric | Degasification |
| Membrane distillation (MD) | - | Temperature and concentration gradient | Highly porous, symmetric | Desalination |
| Pervaporation (PV) | - | Transmembrane fugacity difference | Nonporous hydrophilic (CS, SPEEK/PES) * (PDMS/CERAM) * (PEBA/PU) * (PEI/GO) * | Desalination [64,65,66] [67] [68] [69] |
| Membrane WT Process | Water Flux | ECs Flux | References |
|---|---|---|---|
| MF | Yes | No | [70,71,72] |
| UF | Yes | No | [59,60] |
| NF | Yes | No | [61,73,74,75,76,77] |
| FO | Yes | No | [62,78,79,80] |
| RO | Yes | No | [60,61,63,74,81,82] |
| ED | No | No | Other |
| EDI | No | No | Other |
| MDg | No | No | Other |
| MD | Yes | No | |
| PV | Yes | Yes | [57,68,69,83,84,85,86,87] |
| Perspective | Membrane Separation Based on Pore Size | Separation Based on Target Transport of ECs | Note |
|---|---|---|---|
| Rate of the process | ++ | -- | (a) |
| Economics | ++ | -- | (b) |
| Available on the market | + | - | (c) |
| Number of membrane types needed | + | - | (d) |
| Prediction | -- | ++ | (e) |
| Treated water | -- | ++ | (f) |
| Fouling | - | + | (g) |
| Pressure | - | + | (h) |
| Parameters | Units | Ethinyl Estradiol | Ibuprofen |
|---|---|---|---|
| Water solubility | g/L | 1.13 × 10−2 | 2.10 × 10−2 |
| Ethanol solubility | g/L | 50 | 60 |
| c in particular case | g/L | 5.10 × 10−8 | 9.90 × 10−6 |
| Reference | - | [77] | [15] |
| Amount of water from particular case extractable by 1 L of Ethanol | m3 | 9.8 × 10−5 | 6060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kárászová, M.; Bourassi, M.; Gaálová, J. Membrane Removal of Emerging Contaminants from Water: Which Kind of Membranes Should We Use? Membranes 2020, 10, 305. https://doi.org/10.3390/membranes10110305
Kárászová M, Bourassi M, Gaálová J. Membrane Removal of Emerging Contaminants from Water: Which Kind of Membranes Should We Use? Membranes. 2020; 10(11):305. https://doi.org/10.3390/membranes10110305
Chicago/Turabian StyleKárászová, Magda, Mahdi Bourassi, and Jana Gaálová. 2020. "Membrane Removal of Emerging Contaminants from Water: Which Kind of Membranes Should We Use?" Membranes 10, no. 11: 305. https://doi.org/10.3390/membranes10110305