Invasive Diagnostic Procedures from Bronchoscopy to Surgical Biopsy—Optimization of Non-Small Cell Lung Cancer Samples for Molecular Testing
Abstract
:1. Introduction
2. Optimizing Tissue Sampling for LC Diagnosis, Subtyping and Molecular Analysis
2.1. Cryobiopsy for Endoscopy Visible Tumor Lesions and Transbronchial Cryobiopsy for Peripheral Tumor Lesions—Application of Molecular Tests
2.2. Radial Probe—Endobronchial Ultrasound with a Guide Sheath in the Diagnosis of Peripheral Lung Lesions
RP-EBUS-GS—Guided Endobronchial Ultrasound in the Era of Molecular Testing of Tumor Tissue
2.3. Endobronchial Ultrasound (EBUS) Transbronchial Needle Aspiration
RP-EBUS-GS—Guided Endobronchial Ultrasound in the Era of Molecular Testing of Tumor Tissue
3. PD-L1 Testing on Small Biopsies and Cytology Samples
4. Mediastinoscopy and Molecular Testing
5. Transthoracic Needle Aspiration/Biopsy in the Era of Molecular Testing of Lung Tumors
6. Thoracocentesis and Thoracoscopy
7. The Importance of Rebiopsy in the Era of Molecular Therapy for LC
7.1. Histological Changes in LC
7.2. Acquired Resistance and Rebiopsy in the Era of Molecular Testing
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenhalgh, J.; Dwan, K.; Boland, A.; Bates, V.; Vecchio, F.; Dundar, Y.; Jain, P.; Green, J. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst. Rev. 2016, 2016, CD010383. [Google Scholar] [CrossRef] [PubMed]
- Holleman, M.S.; van Tinteren, H.; Groen, H.J.M.; Al, M.J.; Uyl-de Groot, C.A. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: A network meta-analysis. Onco Targets Ther. 2019, 12, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fong, T.; Xia, Z.; Zhang, J.; Luo, P. The efficacy and safety of ALK inhibitors in the treatment of ALK-positive non-small cell lung cancer: A network meta-analysis. Cancer Med. 2018, 7, 4993–5005. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.J.; Kim, D.W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yuan, M.; Li, X.; Chen, L.; Yang, J.; Zhao, X.; Ma, W.; Xin, J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: A systematic review with meta-analysis. Lung Cancer 2013, 81, 1–10. [Google Scholar] [CrossRef]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef]
- Friedlaender, A.; Drilon, A.; Weiss, G.J.; Banna, G.L.; Addeo, A. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer Treat. Rev. 2020, 85, 101978. [Google Scholar] [CrossRef]
- Kim, R.; Keam, B.; Hahn, S.; Ock, C.-Y.; Kim, M.; Kim, T.M.; Kim, D.W.; Heo, D.S. First-line Pembrolizumab Versus Pembrolizumab Plus Chemotherapy Versus Chemotherapy Alone in Non–small-cell Lung Cancer: A Systematic Review and Network Meta-analysis. Clin. Lung Cancer 2019, 20, 331–338.e4. [Google Scholar] [CrossRef]
- Kirita, K.; Izumo, T.; Matsumoto, Y.; Hiraishi, Y.; Tsuchida, T. Bronchoscopic Re-biopsy for Mutational Analysis of Non-small Cell Lung Cancer. Lung 2016, 194, 371–378. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of American pathologists/international association for the. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer Version 6; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2019. [Google Scholar]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, K. Advancements in next-generation sequencing for diagnosis and treatment of non-small-cell lung cancer. Chronic Dis. Transl. Med. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chung, F.T. Central airway tumors: Interventional bronchoscopy in diagnosis and management. J. Thorac. Dis. 2016, 8, E1168–76. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, M.M.; Herth, F.; Yasufuku, K.; Shepherd, R.W.; Yarmus, L.; Chawla, M.; Lamb, C.; Casey, K.R.; Patel, S.; Silvestri, G.A.; et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration CHEST guideline and expert panel report. Chest 2016, 149, 816–835. [Google Scholar] [CrossRef]
- Herth, F. Bronchoscopic techniques in diagnosis and staging of lung cancer. Breathe 2011, 7, 324–337. [Google Scholar] [CrossRef]
- Arimura, K.; Tagaya, E.; Akagawa, H.; Nagashima, Y.; Shimizu, S.; Atsumi, Y.; Sato, A.; Kanzaki, M.; Kondo, M.; Takeyama, K.; et al. Cryobiopsy with endobronchial ultrasonography using a guide sheath for peripheral pulmonary lesions and DNA analysis by next generation sequencing and rapid on-site evaluation. Respir. Investig. 2019, 57, 150–156. [Google Scholar] [CrossRef]
- Hetzel, J.; Eberhardt, R.; Herth, F.J.; Petermann, C.; Reichle, G.; Freitag, L.; Dobbertin, I.; Franke, K.J.; Stanzel, F.; Beyer, T.; et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: A multicentre trial. Eur. Respir. J. 2012, 39, 685–690. [Google Scholar] [CrossRef]
- Rubio, E.R.; le, S.R.; Whatley, R.E.; Boyd, M.B. Cryobiopsy: Should this be used in place of endobronchial forceps biopsies? Biomed. Res. Int. 2013, 2013, 730574. [Google Scholar] [CrossRef]
- Casadevall, D.; Clavé, S.; Taus, Á.; Hardy-Werbin, M.; Rocha, P.; Lorenzo, M.; Menéndez, S.; Salido, M.; Albanell, J.; Pijuan, L.; et al. Heterogeneity of Tumor and Immune Cell PD-L1 Expression and Lymphocyte Counts in Surgical NSCLC Samples. Clin. Lung Cancer 2017, 18, 682–691.e5. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Simoff, M.J.; Wagner, O.J.; Lavin, J. Biopsy frequency and complications among lung cancer patients in the United States. Lung Cancer Manag. 2020, 9, LMT40. [Google Scholar] [CrossRef]
- Simon, M.; Simon, I.; Tent, P.A.; Todea, D.A.; Haranguș, A. Cryobiopsy in lung cancer diagnosis—A literature review. Medicina 2021, 57, 393. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.P.; Mehta, A.C. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 131S–148S. [Google Scholar] [CrossRef] [PubMed]
- Nasu, S.; Okamoto, N.; Suzuki, H.; Shiroyama, T.; Tanaka, A.; Samejima, Y.; Kanai, T.; Noda, Y.; Morita, S.; Morishita, N.; et al. Comparison of the utilities of cryobiopsy and forceps biopsy for peripheral lung cancer. Anticancer Res. 2019, 39, 5683–5688. [Google Scholar] [CrossRef]
- Imabayashi, T.; Uchino, J.; Yoshimura, A.; Chihara, Y.; Tamiya, N.; Kaneko, Y.; Yamada, T.; Takayama, K. Safety and usefulness of cryobiopsy and stamp cytology for the diagnosis of peripheral pulmonary lesions. Cancers 2019, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, H.; Kirita, K.; Naito, T.; Nomura, S.; Ishibashi, M.; Matsuzawa, R.; Hisakane, K.; Usui, Y.; Matsumoto, S.; Yoh, K.; et al. Feasibility and utility of transbronchial cryobiopsy in precision medicine for lung cancer: Prospective single-arm study. Cancer Sci. 2020, 111, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Bevill, B.T.; Huang, J.; Brooks, M.; Choi, Y.; Kennedy, G.; Lofaro, L.; Chen, A.; Rivera, M.P.; Tanner, N.T.; et al. An Evaluation of Diagnostic Yield from Bronchoscopy: The Impact of Clinical/Radiographic Factors, Procedure Type, and Degree of Suspicion for Cancer. Chest 2020, 157, 1656–1664. [Google Scholar] [CrossRef]
- Ahn, J.H. An update on the role of bronchoscopy in the diagnosis of pulmonary disease. Yeungnam Univ. J. Med. 2020, 37, 253–261. [Google Scholar]
- Kurimoto, N.; Murayama, M.; Yoshioka, S.; Nishisaka, T. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest 2002, 122, 1887–1894. [Google Scholar] [CrossRef]
- Kikuchi, E.; Yamazaki, K.; Sukoh, N.; Kikuchi, J.; Asahina, H.; Imura, M.; Onodera, Y.; Kurimoto, N.; Kinoshita, I.; Nishimura, M. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur. Respir. J. 2004, 24, 533–537. [Google Scholar] [CrossRef]
- Moon, S.M.; Choe, J.; Jeong, B.H.; Um, S.W.; Kim, H.; Jung Kwon, O.; Lee, K. Diagnostic performance of radial probe endobronchial ultrasound without a guide-sheath and the feasibility of molecular analysis. Tuberc. Respir. Dis. 2019, 82, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Eom, J.S.; Kim, A.R.; Lee, C.H.; Lee, G.; Jo, E.J.; Kim, M.H.; Mok, J.H.; Lee, K.; Kim, K.U.; et al. Molecular analysis of small tissue samples obtained via transbronchial lung biopsy using radial probe endobronchial ultrasound. PLoS ONE 2019, 14, e0212672. [Google Scholar] [CrossRef] [PubMed]
- Guisier, F.; Salaün, M.; Lachkar, S.; Lamy, A.; Piton, N.; Obstoy, B.; Sabourin, J.-C.; Thiberville, L. Molecular analysis of peripheral non-squamous non-small cell lung cancer sampled by radial EBUS. Respirology 2016, 21, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Folch, E.; Jantz, M.; Mehta, H.J.; Majid, A.; Fernandez-Bussy, S. Adequacy of Samples Obtained by Endobronchial Ultrasound with Transbronchial Needle Aspiration for Molecular Analysis in Patients with Non-Small Cell Lung Cancer. Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2018, 15, 1205–1216. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; De Biase, D.; Valentini, I.; Casadei, G.; Paioli, D.; Ferrari, F.; Gordini, G.; Patelli, M.; et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration with and without rapid on-site evaluation for lung cancer genotyping. Chest 2015, 148, 1430–1437. [Google Scholar] [CrossRef]
- Dooms, C.; Vander Borght, S.; Yserbyt, J.; Testelmans, D.; Wauters, E.; Nackaerts, K.; Vansteenkiste, J.; Verbeken, E.; Weynand, B. A randomized clinical trial of flex 19G needles versus 22G needles for endobronchial ultrasonography in suspected lung cancer. Respiration 2018, 96, 275–282. [Google Scholar] [CrossRef]
- Garrison, G.; Leclair, T.; Balla, A.; Wagner, S.; Butnor, K.; Anderson, S.R.; Kinsey, C.M. Use of an Additional 19-GEBUS-TBNANeedle Increases the Diagnostic Yield of EBUS-TBNA. J. Bronchol. Interv. Pulmonol. 2018, 25, 269–273. [Google Scholar] [CrossRef]
- Fernandez-Bussy, S.; Labarca, G.; Pires, Y.; Caviedes, I.; Burotto, M. Análisis moleculares de EGFR, mutación de resistencia al EGFR, ALK y ROS1 en muestras obtenidas mediante PATB-USEB en Chile. Arch. Bronconeumol. 2017, 53, 172–174. [Google Scholar] [CrossRef]
- Cicek, T.; Ozturk, A.; Yılmaz, A.; Aktas, Z.; Demirag, F.; Akyurek, N. Adequacy of EBUS-TBNA specimen for mutation analysis of lung cancer. Clin. Respir. J. 2019, 13, 92–97. [Google Scholar] [CrossRef]
- Xie, F.; Zheng, X.; Mao, X.; Zhao, R.; Ye, J.; Zhang, Y.; Sun, J. Next-Generation Sequencing for Genotyping of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Samples in Lung Cancer. Ann. Thorac. Surg. 2019, 108, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R. The IASLC Atlas of PD-L1 Immunohistochemistry (IHC) Testing in Lung Cancer Released. 2017, pp. 2–3. Available online: https://www.iaslc.org/research-education/publications-resources-guidelines/iaslc-atlas-pd-l1-testing-lung-cancer (accessed on 1 May 2023).
- Nakamura, S.; Hayashi, K.; Imaoka, Y.; Kitamura, Y.; Akazawa, Y.; Tabata, K.; Groen, R.; Tsuchiya, T.; Yamasaki, N.; Nagayasu, T.; et al. Intratumoral heterogeneity of programmed cell death ligand-1 expression is common in lung cancer. PLoS ONE 2017, 12, e0186192. [Google Scholar] [CrossRef] [PubMed]
- Bigras, G.; Mairs, S.; Swanson, P.E.; Morel, D.; Lai, R.; Izevbaye, I. Small Biopsies Misclassify up to 35% of PD-L1 Assessments in Advanced Lung Non-Small Cell Lung Carcinomas. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 701–708. [Google Scholar] [CrossRef]
- Kitazono, S.; Fujiwara, Y.; Tsuta, K.; Utsumi, H.; Kanda, S.; Horinouchi, H.; Nokihara, H.; Yamamoto, N.; Sasada, S.; Watanabe, S.; et al. Reliability of Small Biopsy Samples Compared with Resected Specimens for the Determination of Programmed Death-Ligand 1 Expression in Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2015, 16, 385–390. [Google Scholar] [CrossRef]
- Sakata, K.K.; Midthun, D.E.; Mullon, J.J.; Kern, R.M.; Nelson, D.R.; Edell, E.S.; Schiavo, D.N.; Jett, J.R.; Aubry, M.C. Comparison of Programmed Death Ligand-1 Immunohistochemical Staining Between Endobronchial Ultrasound Transbronchial Needle Aspiration and Resected Lung Cancer Specimens. Chest 2018, 154, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, Q.; Yu, Z.; Dong, H.; Li, X.; Chen, Q.; Hu, B.; Li, H.; Miao, J. Clinical application of ultrasound-guided mediastinal lymph node biopsy through cervical mediastinoscopy. Thorac. Cancer 2021, 12, 297–303. [Google Scholar] [CrossRef]
- Schneider, F.; Smith, M.A.; Lane, M.C.; Pantanowitz, L.; Dacic, S.; Ohori, N.P. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am. J. Clin. Pathol. 2015, 143, 193–200. [Google Scholar] [CrossRef]
- Transthoracic Fine Needle Aspiration (FNA). Eurocytology. Available online: https://www.eurocytology.eu/course/respiratory-tract/cell-sampling-and-preparation-methods/transthoracic-fine-needle-aspiration-fna/ (accessed on 19 May 2023).
- Han, H.S.; Eom, D.W.; Kim, J.H.; Kim, K.H.; Shin, H.M.; An, J.Y.; Lee, K.M.; Choe, K.H.; Lee, K.H.; Kim, S.T.; et al. EGFR mutation status in primary lung adenocarcinomas and corresponding metastatic lesions: Discordance in pleural metastases. Clin. Lung Cancer 2011, 12, 380–386. [Google Scholar] [CrossRef]
- Murthy, P.; Ekeke, C.N.; Russell, K.L.; Butler, S.C.; Wang, Y.; Luketich, J.D.; Soloff, A.C.; Dhupar, R.; Lotze, M.T. Making cold malignant pleural effusions hot: Driving novel immunotherapies. Oncoimmunology 2019, 8, e1554969. [Google Scholar] [CrossRef]
- Aggarwal, C.; Haas, A.R.; Metzger, S.; Aguilar, L.K.; Aguilar-Cordova, E.; Manzanera, A.G.; Gómez-Hernández, G.; Katz, S.I.; Alley, E.W.; Evans, T.L.; et al. Phase I Study of Intrapleural Gene-Mediated Cytotoxic Immunotherapy in Patients with Malignant Pleural Effusion. Mol. Ther. 2018, 26, 1198–1205. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Y.; Hu, Z.; Wu, N.; Nie, X.; Xia, Y.; Han, Y.; Li, Q.; Zhu, G.; Bai, C. Malignant pleural effusion supernatants are substitutes for metastatic pleural tumor tissues in EGFR mutation test in patients with advanced lung adenocarcinoma. PLoS ONE 2014, 9, e89946. [Google Scholar] [CrossRef] [PubMed]
- Ofiara, L.M.; Navasakulpong, A.; Beaudoin, S.; Gonzalez, A.V. Optimizing tissue sampling for the diagnosis, subtyping and molecular analysis of lung cancer. Front. Oncol. 2014, 4, 253. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J. Molecular classification of non-small-cell lung cancer: Diagnosis, individualized treatment, and prognosis. Front. Med. 2013, 7, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Jekunen, A.P. Role of Rebiopsy in Relapsed Non-Small Cell Lung Cancer for Directing Oncology Treatments. J. Oncol. 2015, 2015, 809835. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef]
- Mondoni, M.; Rinaldo, R.F.; Carlucci, P.; Terraneo, S.; Saderi, L.; Centanni, S.; Sotgiu, G. Bronchoscopic sampling techniques in the era of technological bronchoscopy. Pulmonology 2022, 28, 461–471. [Google Scholar] [CrossRef]





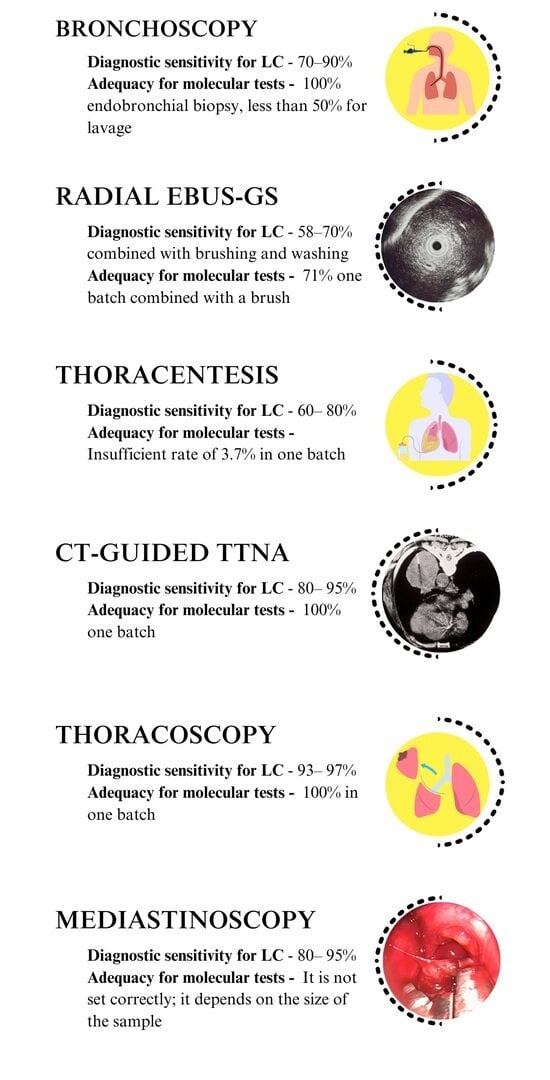
| Diagnostic Procedure | Sample Type | Diagnostic Sensitivity | Adequacy for Molecular Tests |
|---|---|---|---|
| Bronchoscopy | Endobronchial biopsy | 70–90% (if the lesion is visible) | 100% endobronchial biopsy, less than 50% for lavage |
| Brush cytology | The sensitivity increases when combined with bronchial biopsy and lavage | ||
| Lavage cytology | |||
| Radial EBUS-GS (for peripheral lesions larger than 2 cm) | Transbronchial biopsy | 58–70% combined with brushing and washing | 71% one batch combined with a brush |
| Radial EBUS-GS (for peripheral lesions 2 cm and smaller) | Brush cytology | ||
| Lavage cytology | |||
| Mediastinoscopy | Biopsy | 80–95% | It is not set correctly; it depends on the size of the sample |
| CT-guided TTNA | Core needle biopsy | 80–95% | 100% one batch |
| Needle aspiration Cytology | |||
| Thoracentesis | Cytology | 60–80% | Insufficient rate of 3.7% in one batch |
| Thoracoscopy | Biopsy | 93–97% | 100% in one batch |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalić, N.; Lovrenski, A.; Ilić, M.; Ivanov, O.; Bojović, M.; Lalić, I.; Popević, S.; Stjepanović, M.; Janjić, N. Invasive Diagnostic Procedures from Bronchoscopy to Surgical Biopsy—Optimization of Non-Small Cell Lung Cancer Samples for Molecular Testing. Medicina 2023, 59, 1723. https://doi.org/10.3390/medicina59101723
Lalić N, Lovrenski A, Ilić M, Ivanov O, Bojović M, Lalić I, Popević S, Stjepanović M, Janjić N. Invasive Diagnostic Procedures from Bronchoscopy to Surgical Biopsy—Optimization of Non-Small Cell Lung Cancer Samples for Molecular Testing. Medicina. 2023; 59(10):1723. https://doi.org/10.3390/medicina59101723
Chicago/Turabian StyleLalić, Nensi, Aleksandra Lovrenski, Miroslav Ilić, Olivera Ivanov, Marko Bojović, Ivica Lalić, Spasoje Popević, Mihailo Stjepanović, and Nataša Janjić. 2023. "Invasive Diagnostic Procedures from Bronchoscopy to Surgical Biopsy—Optimization of Non-Small Cell Lung Cancer Samples for Molecular Testing" Medicina 59, no. 10: 1723. https://doi.org/10.3390/medicina59101723