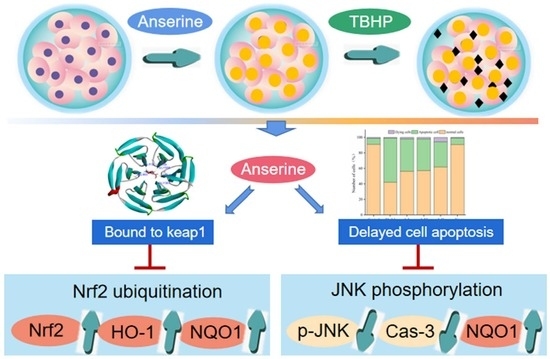
The Preventive Mechanism of Anserine on Tert-Butyl Hydroperoxide-Induced Liver Injury in L-02 Cells via Regulating the Keap1-Nrf2 and JNK-Caspase-3 Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Effects of TBHP on the Survival Rate of L-02 Cells
2.2. Protective Effect of Anserine on L-02 Cell Injury
2.3. Effect of Anserine on Biochemical Indicators in TBHP-Induced Damaged L-02 Cells
2.4. The Positive Regulation of Anserine on the Keap1-Nrf2 Signaling Pathway in L-02 Cells
2.5. Inhibition of L-02 Cell Apoptosis by Anserine
2.6. Effect of Anserine on the JNK-Bcl-2-Caspase-3 Signaling Pathway in L-02 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Establishment of TBHP Induced L-02 Cell Injury Model
4.4. Cytotoxicity and Proliferation Assay
4.5. Cytoprotective Effect of Anserine
4.6. Measurement of ALT, AST, ROS, and GSH in TBHP-Induced L-02 Cells
4.7. Fluorescence Observation of L-02 Nucleus Using DAPI Stain
4.8. Molecular Docking
4.9. Determination of Apoptosis
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Author Correction: Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dudley, S.C., Jr. Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M. Nrf2 for cardiac protection: Pharmacological options against oxidative stress. Trends Pharmacol. Sci. 2021, 42, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Tie, H.; Tian, W.; Zhao, Y.; Qin, L.; Guo, S.; Li, Q.; Bao, C. Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J. Biochem. Mol. Toxicol. 2023, 37, e23368. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, J.; Yu, T.; Wang, M.; Jin, L.; Liang, S.; Luo, W.; Wang, Y.; Li, G.; Liang, G. Diacerein protects liver against APAP-induced injury via targeting JNK and inhibiting JNK-mediated oxidative stress and apoptosis. Biomed. Pharmacother. 2022, 149, 112917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, W.; Gu, X.; Miao, C.; Feng, L.; Shen, Q.; Liu, X.; Zhang, X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022, 8, 162. [Google Scholar] [CrossRef]
- Sadasivam, N.; Kim, Y.J.; Radhakrishnan, K.; Kim, D.K. Oxidative Stress, Genomic Integrity, and Liver Diseases. Molecules 2022, 27, 3159. [Google Scholar] [CrossRef]
- Ruart, M.; Chavarria, L.; Campreciós, G.; Suárez-Herrera, N.; Montironi, C.; Guixé-Muntet, S.; Bosch, J.; Friedman, S.L.; Garcia-Pagán, J.C.; Hernández-Gea, V. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol. 2019, 70, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, M.; Lee, D.; Law, C.T.; Wei, L.; Tsang, F.H.; Chin, D.W.; Cheng, C.L.; Lee, J.M.; Ng, I.O.; et al. Histone chaperone FACT complex mediates oxidative stress response to promote liver cancer progression. Gut 2020, 69, 329–342. [Google Scholar] [CrossRef]
- SG, B.G.; Tsukui, T.; Fuda, H.; Minami, Y.; Gowda, D.; Chiba, H.; Hui, S.P. Docosahexaenoic Acid Esters of Hydroxy Fatty Acid Is a Novel Activator of NRF2. Int. J. Mol. Sci. 2021, 22, 7598. [Google Scholar]
- SG, B.G.; Fuda, H.; Tsukui, T.; Chiba, H.; Hui, S.P. Discovery of Eicosapentaenoic Acid Esters of Hydroxy Fatty Acids as Potent Nrf2 Activators. Antioxidants 2020, 9, 397. [Google Scholar]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Xu, G.B.; Xiao, Y.H.; Zhang, Q.Y.; Zhou, M.; Liao, S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, Q.; Wang, Z.; Lu, C.; Zhou, J.; Li, Y.; Ming, T.; Zhang, Z.; Su, X. Spatial distribution of gut microbiota in mice during the occurrence and remission of hyperuricemia. J. Sci. Food Agric. 2023, 103, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, N.; Wang, K.; Zhang, L.; Li, D.; Wang, Z.; Xu, G.; Liu, Y.; Xu, Q. Rosamultin from Potentilla anserine L. exhibits nephroprotection and antioxidant activity by regulating the reactive oxygen species/C/EBP homologous protein signaling pathway. Phytother. Res. 2021, 35, 6343–6358. [Google Scholar] [CrossRef]
- Yamano, E.; Tanaka, M.; Ishii, A.; Tsuruoka, N.; Abe, K.; Watanabe, Y. Effects of chicken essence on recovery from mental fatigue in healthy males. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2013, 19, 540–547. [Google Scholar]
- Wei, S.; Li, C.; Luo, X.; Yang, L.; Yu, L.; Wang, Q.; Meng, Z.X.; Wang, T.; Chen, Y. Intermittent protein restriction protects islet β cells and improves glucose homeostasis in diabetic mice. Sci. Bull. 2022, 67, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Lei, C.; Li, H.; Inamura, N.; Shiotani, S.; Yanai, N.; Sato, K.; Sakurai, K.; Hisatsune, T. Anserine, HClO-scavenger, protected against cognitive decline in individuals with mild cognitive impairment. Aging 2021, 13, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ji, H.; Song, W.; Zhang, D.; Su, W.; Liu, S. Anserine beneficial effects in hyperuricemic rats by inhibiting XOD, regulating uric acid transporter and repairing hepatorenal injury. Food Funct. 2022, 13, 9434–9442. [Google Scholar] [CrossRef] [PubMed]
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. tBHP treatment as a model for cellular senescence and pollution-induced skin aging. Mech. Ageing Dev. 2020, 190, 111318. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, H.; Gong, W.; Cao, F.; Wu, T.; Hu, F. Plumbagin protects H9c2 cardiomyocytes against TBHP-induced cytotoxicity by alleviating ROS-induced apoptosis and modulating autophagy. Exp. Ther. Med. 2022, 24, 501. [Google Scholar] [CrossRef]
- Taniguchi, K.; Igaki, T. Sas-Ptp10D shapes germ-line stem cell niche by facilitating JNK-mediated apoptosis. PLoS Genet. 2023, 19, e1010684. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, X.; Chaggan, C.; Liao, Z.; In Wong, K.; He, F.; Singh, S.; Loomba, R.; Karin, M.; Witztum, J.L.; et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science 2020, 367, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Kim, J.H.; Che, D.N.; Kang, H.J.; Shin, J.Y.; Hao, S.; Park, J.H.; Wang, F.; Lee, Y.J.; Jang, S.I. Kushenol C Prevents Tert-Butyl Hydroperoxide and Acetaminophen-Induced Liver Injury. Molecules 2021, 26, 1635. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects t-butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 2019, 55, 92–104. [Google Scholar] [CrossRef]
- Hakim, A.; Moll, M.; Brancale, J.; Liu, J.; Lasky-Su, J.A.; Silverman, E.K.; Vilarinho, S.; Jiang, Z.G.; Pita-Juárez, Y.H.; Vlachos, I.S.; et al. Genetic Variation in the Mitochondrial Glycerol-3-Phosphate Acyltransferase Is Associated With Liver Injury. Hepatology 2021, 74, 3394–3408. [Google Scholar] [CrossRef]
- Liu, B.; Ding, C.; Tang, W.; Zhang, C.; Gu, Y.; Wang, Z.; Yu, T.; Li, Z. Hepatic ROS Mediated Macrophage Activation Is Responsible for Irinotecan Induced Liver Injury. Cells 2022, 11, 3791. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.B.; Wang, Z.; An, W.J. Protection of Icariin Against Hydrogen Peroxide-Induced MC3T3-E1 Cell Oxidative Damage. Orthop. Surg. 2021, 13, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, L.; Fu, Y.; Wang, W.; Wang, P.; Zhou, F. Ginnalin A Binds to the Subpockets of Keap1 Kelch Domain To Activate the Nrf2-Regulated Antioxidant Defense System in SH-SY5Y Cells. ACS Chem. Neurosci. 2021, 12, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Chen, J.; Cao, J.; Xiao, J.; Li, X.; Sun, C. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, F.; Yang, W.; Ren, J.; Ge, W.; Yang, P. Apoptosis as an underlying mechanism in lymphocytes induced by riboflavin and ultraviolet light. Transfus. Apher. Sci. 2020, 59, 102899. [Google Scholar] [CrossRef]
- Liang, J.; Cao, R.; Wang, X.; Zhang, Y.; Wang, P.; Gao, H.; Li, C.; Yang, F.; Zeng, R.; Wei, P.; et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017, 27, 329–351. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.K.; Kini, S.G.; Garg, V.; Agrawal, S.; Tomar, P.K.; Pathak, P.; Chaudhary, A.; Gupta, P.; Malik, A. JNK pathway signaling: A novel and smarter therapeutic targets for various biological diseases. Future Med. Chem. 2015, 7, 2065–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.L.; Hai, C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]







| Concentration (μmol/L) | Cell Survival Rate (%) | ||||
|---|---|---|---|---|---|
| 2 h | 4 h | 8 h | 12 h | 24 h | |
| 0 | 100 ± 4.17 | 100 ± 1.58 | 100 ± 1.06 | 100 ± 1.46 | 100 ± 1.21 |
| 200 | 74.04 ± 3.91 | 71.82 ± 1.14 | 66.77 ± 1.21 | 49.35 ± 1.24 | 30.04 ± 0.81 |
| 400 | 65.79 ± 5.43 | 57.85 ± 0.71 | 44.13 ± 1.13 | 40.76 ± 1.28 | 27.16 ± 0.95 |
| 600 | 49.02 ± 3.76 | 43.86 ± 1.11 | 36.91 ± 1.12 | 37.62 ± 1.25 | 23.61 ± 0.63 |
| 800 | 44.86 ± 4.12 | 38.61 ± 0.81 | 35.44 ± 1.01 | 31.79 ± 1.28 | 21.03 ± 0.25 |
| 1000 | 39.36 ± 2.92 | 30.81 ± 0.73 | 28.68 ± 1.24 | 25.09 ± 0.76 | 21.48 ± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Luo, J.; Ji, H.; Song, W.; Zhang, D.; Su, W.; Liu, S. The Preventive Mechanism of Anserine on Tert-Butyl Hydroperoxide-Induced Liver Injury in L-02 Cells via Regulating the Keap1-Nrf2 and JNK-Caspase-3 Signaling Pathways. Mar. Drugs 2023, 21, 477. https://doi.org/10.3390/md21090477
Chen M, Luo J, Ji H, Song W, Zhang D, Su W, Liu S. The Preventive Mechanism of Anserine on Tert-Butyl Hydroperoxide-Induced Liver Injury in L-02 Cells via Regulating the Keap1-Nrf2 and JNK-Caspase-3 Signaling Pathways. Marine Drugs. 2023; 21(9):477. https://doi.org/10.3390/md21090477
Chicago/Turabian StyleChen, Ming, Jing Luo, Hongwu Ji, Wenkui Song, Di Zhang, Weiming Su, and Shucheng Liu. 2023. "The Preventive Mechanism of Anserine on Tert-Butyl Hydroperoxide-Induced Liver Injury in L-02 Cells via Regulating the Keap1-Nrf2 and JNK-Caspase-3 Signaling Pathways" Marine Drugs 21, no. 9: 477. https://doi.org/10.3390/md21090477