The Effect of Low-Processing Temperature on the Physicochemical and Mechanical Properties of Bovine Hydroxyapatite Bone Substitutes
Abstract
:1. Introduction
2. Materials and Methods
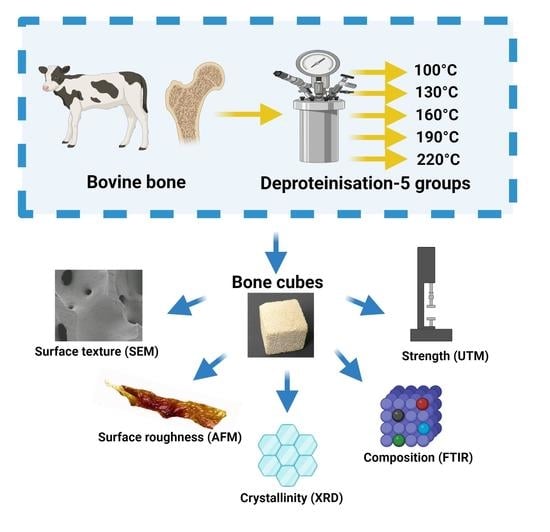
2.1. Sample Preparation and Heat Treatment
2.2. Scanning Electron Microscopy (SEM) Imaging
2.3. Surface Topography Imaging Using Atomic Force Microscopy (AFM)
2.4. Compressive Strength
2.5. Determining the Residual Organic Content by Thermogravimetric Analysis (TGA)
2.6. Chemical Composition and Crystallinity Analysis by Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Crystallinity Analysis by X-ray Diffraction
2.8. Statistical Analyses
3. Results
3.1. Surface Morphology of Bovine Bone Graft Materials as Determined by SEM
3.2. Surface Topography and Mechanical Properties Established by AFM
3.3. Compressive Strength
3.4. Residual Organic Content
3.5. Chemical Composition and Crystallinity by FTIR
3.6. Crystallinity Utilising XRD
4. Discussion
4.1. Changes in Surface Texture and Topography with Increased Temperature
4.2. Relation between Bone Composition and Mechanical Properties
4.3. Effect of Temperature on the Organic Component
4.4. Effect of Temperature on the Inorganic Component
4.5. Effect of Temperature on the Atomic Substitution and Bone Composition Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Inj. Int. J. Care Inj. 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. 1), S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Swan, M.C.; Goodacre, T.E.E. Morbidity at the iliac crest donor site following bone grafting of the cleft alveolus. Br. J. Oral Maxillofac. Surg. 2006, 44, 129–133. [Google Scholar] [PubMed]
- Sasso, R.C.; Williams, J.I.; Di Masi, N.; Meyer, P.R. Postoperative drains at the donor sites of iliac-crest bone grafts—A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J. Bone Jt. Surg. 1998, 80, 631–635. [Google Scholar]
- Castiglioni, S.; Cazzaniga, A.; Locatelli, L.; Maier, J.A.M. Silver nanoparticles in orthopedic applications: New insights on their effects on osteogenic cells. Nanomaterials 2017, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Kalk, W.W.; Raghoebar, G.M.; Jansma, J.; Boering, G. Morbidity from iliac crest bone harvesting. J. Oral Maxillofac. Surg. 1996, 54, 1424–1429; discussion 1430. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42 (Suppl. 2), S3–S15. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.; Lin, X. Histological outcomes of sinus augmentation for dental implants with calcium phosphate or deproteinized bovine bone: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 1471–1477. [Google Scholar] [CrossRef]
- Yildirim, M.; Spiekermann, H.; Biesterfeld, S.; Edelhoff, D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss® in combination with venous blood. Clin. Oral Implant. Res. 2000, 11, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Conz, M.B.; Granjeiro, J.M.; Gde, A.S. Physicochemical characterization of six commercial hydroxyapatites for medical-dental applicatons as bone graft. J. Appl. Oral Sci. 2005, 13, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Athanasiou, K.A.; Zhu, C.-F.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, T.; Conz, M.B.; Barros, T.C.; Sena, L.Á.D.; Soares, G.D.A.; Granjeiro, J.M. Physicochemical characterization of two deproteinized bovine xenografts. Braz. Oral Res. 2008, 22, 5–10. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.-P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef]
- Heinz Lussi, P.G. Process For Preparing High Purity Bone Mineral; Geistlich Sohne AG fur Chemische Industrie: Schlieren, Switzerland, 1988. [Google Scholar]
- Jayesh, R.S.; Dhinakarsamy, V. Osseointegration. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 1), S226–S229. [Google Scholar]
- Lindhe, J.; Cecchinato, D.; Donati, M.; Tomasi, C.; Liljenberg, B. Ridge preservation with the use of deproteinized bovine bone mineral. Clin. Oral Implant. Res. 2014, 25, 786–790. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Kostopoulos, L.; Mardas, N.; Randel Nyengaard, J.; Karring, T. Deproteinized bovine bone used as an adjunct to guided bone augmentation: An experimental study in the rat. Clin. Implant Dent. Relat. Res. 2001, 3, 156–165. [Google Scholar] [CrossRef]
- Donos, N.; Lang, N.P.; Karoussis, I.K.; Bosshardt, D.; Tonetti, M.; Kostopoulos, L. Effect of GBR in combination with deproteinized bovine bone mineral and/or enamel matrix proteins on the healing of critical-size defects. Clin. Oral Implant. Res. 2004, 15, 101–111. [Google Scholar] [CrossRef]
- Slotte, C.; Lundgren, D. Augmentation of calvarial tissue using non-permeable silicone domes and bovine bone mineral. An experimental study in the rat. Clin. Oral Implant. Res. 1999, 10, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Suter, A.J.; Molteno, A.C.B.; Bevin, T.H.; Fulton, J.D.; Herbison, G.P. Long term follow up of bone derived hydroxyapatite orbital implants. Br. J. Ophthalmol. 2002, 86, 1287–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.M.; Duncan, W.J.; Coates, D.E. Attributes of Bio-Oss((R)) and Moa-Bone((R)) graft materials in a pilot study using the sheep maxillary sinus model. J. Periodontal Res. 2018, 53, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Baryosef, O. States of preservation of bones from prehistoric sites in the near-east—A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Pienkowski, D.; Doers, T.M.; Monier-Faugere, M.-C.; Geng, Z.; Camacho, N.P.; Boskey, A.L.; Malluche, H.H. Calcitonin alters bone quality in beagle dogs. J. Bone Miner. Res. 1997, 12, 1936–1943. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behavior and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Peters, F.; Schwarz, K.; Epple, M. The structure of bone studied with synchrotron X-ray diffraction, X-ray absorption spectroscopy and thermal analysis. Thermochim. Acta 2000, 361, 131–138. [Google Scholar] [CrossRef]
- Pramanik, S.; Hanif, A.S.M.; Pingguan-Murphy, B.; Abu Osman, N.A. Morphological change of heat treated bovine bone: A comparative study. Materials 2012, 6, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Kačarević Željka, P.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.D.; Daniels, P. An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials 2002, 23, 2577–2585. [Google Scholar] [CrossRef]
- Todoh, M.; Tadano, S.; Imari, Y. Effect of heat denaturation of collagen matrix on bone strength. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; Volume 23, pp. 2034–2037. [Google Scholar]
- Wang, X.; Bank, R.A.; Tekoppele, J.M.; Agrawal, C.M. The role of collagen in determining bone mechanical properties. J. Orthop. Res. 2001, 19, 1021–1026. [Google Scholar] [CrossRef]
- Cooper, L.F.; Zhou, Y.; Takebe, J.; Guo, J.; Abron, A.; Holmén, A.; Ellingsen, J.E. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Shalabi, M.; Gortemaker, A.; Hof, M.V.; Jansen, J.; Creugers, N. Implant surface roughness and bone healing: A systematic review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, M.U.; Kim, C.W. Activation of phospholipase D1 by surface roughness of titanium in MG63 osteoblast-like cell. Biomaterials 2006, 27, 5502–5511. [Google Scholar] [CrossRef]
- Ramírez Fernández, M.P.; Gehrke, S.A.; Pérez Albacete Martinez, C.; Calvo Guirado, J.L.; De Aza, P.N. SEM-EDX study of the degradation process of two xenograft materials used in sinus lift procedures. Materials 2017, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Morgan, D.A.; Forwood, M.R. Sterilization of allograft bone: Effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2006, 8, 93–105. [Google Scholar] [CrossRef]
- Revenko, I.; Sommer, F.; Minh, D.T.; Garrone, R.; Franc, J.M. Atomic force microscopy study of the collagen fibre structure. Biol. Cell 1994, 80, 67–69. [Google Scholar] [CrossRef]
- Kindt, J.H.; Thurner, P.J.; Lauer, M.E.; Bosma, B.L.; Schitter, G.; Fantner, G.E.; Izumi, M.; Weaver, J.C.; Morse, D.E.; Hansma, P.K. In situ observation of fluoride-ion-induced hydroxyapatite-collagen detachment on bone fracture surfaces by atomic force microscopy. Nanotechnology 2007, 18, 135102. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, A. Crystallinity and Diagenesis of Sedimentary Apatites. Geochim. Cosmochim. Acta 1990, 54, 2433–2438. [Google Scholar] [CrossRef]
- Asami, A.; Nakamura, M.; Takeuchi, M.; Nakayama, A.; Nakamura, H.; Yoshida, T.; Nagasawa, S.; Hiraoka, B.Y.; Ito, M.; Udagawa, N.; et al. Effects of Heat Treatment of Hydroxyapatite on Osteoblast Differentiation. J. Hard Tissue Biol. 2008, 17, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Piga, G.; Solinas, G.; Thompson, T.; Brunetti, A.; Malgosa, A.; Enzo, S. Is X-ray diffraction able to distinguish between animal and human bones? J. Archaeol. Sci. 2012, 40, 778–785. [Google Scholar] [CrossRef]
- Dal Sasso, G.; Asscher, Y.; Angelini, I.; Nodari, L.; Artioli, G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci. Rep. 2018, 8, 12025. [Google Scholar] [CrossRef]
- Fleet, M.E. The carbonate ion in hydroxyapatite: Recent X-ray and infrared results. Front. Biosci. 2013, 5, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Dumitrescu, C.R.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Iordache, F.; Trusca, R.; Ciocan, L.T.; Ficai, A.; Andronescu, E. Nano-hydroxyapatite vs. xenografts: Synthesis, characterization, and in vitro behavior. Nanomaterials 2021, 11, 2289. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Kijkowska, R.; Bautista, C.; LeGeros, J.P. Synergistic effects of magnesium and carbonate on properties of biological and synthetic apatites. Connect. Tissue Res. 1995, 33, 203–209. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.; Layrolle, P.; Barrere, F.; De Bruijn, J.; Schoonman, J.; Van Blitterswijk, C.A.; De Groot, K. Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro. J. Biomed. Mater. Res. 2001, 56, 208–215. [Google Scholar] [CrossRef]
- Antonia Ressler, A.Ž.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Baig, A.A.; Fox, J.L.; Young, R.A.; Wang, Z.; Hsu, J.; Higuchi, W.I.; Chhettry, A.; Zhuang, H.; Otsuka, M. Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters. Calcif. Tissue Int. 1999, 64, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, M.E.; Miller, L.M.; Burr, D.B. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos. Int. 2008, 19, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Schulze, E.; Witt, M.; Petsch, E.; Biewener, A.; Pompe, W.; Zwipp, H. Collagen type I increases bone remodelling around hydroxyapatite implants in the rat tibia. Cells Tissues Organs 2004, 178, 146–157. [Google Scholar] [CrossRef] [PubMed]







| Temperature (°C) | Pressure (Bar) |
|---|---|
| 100 | 1.01 |
| 130 | 2.69 |
| 160 | 6.22 |
| 190 | 12.93 |
| 220 | 24.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmoneim, D.; Porter, G.C.; Coates, D.E.; Duncan, W.J.; Waddell, J.N.; Hammer, N.; Li, K.C. The Effect of Low-Processing Temperature on the Physicochemical and Mechanical Properties of Bovine Hydroxyapatite Bone Substitutes. Materials 2022, 15, 2798. https://doi.org/10.3390/ma15082798
Abdelmoneim D, Porter GC, Coates DE, Duncan WJ, Waddell JN, Hammer N, Li KC. The Effect of Low-Processing Temperature on the Physicochemical and Mechanical Properties of Bovine Hydroxyapatite Bone Substitutes. Materials. 2022; 15(8):2798. https://doi.org/10.3390/ma15082798
Chicago/Turabian StyleAbdelmoneim, Dina, Gemma Claire Porter, Dawn Elizabeth Coates, Warwick John Duncan, John Neil Waddell, Niels Hammer, and Kai Chun Li. 2022. "The Effect of Low-Processing Temperature on the Physicochemical and Mechanical Properties of Bovine Hydroxyapatite Bone Substitutes" Materials 15, no. 8: 2798. https://doi.org/10.3390/ma15082798