Effects of Montmorillonite and Gentamicin Addition on the Properties of Electrospun Polycaprolactone Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Starting Materials
2.2. Preparation of Samples
2.3. Methods
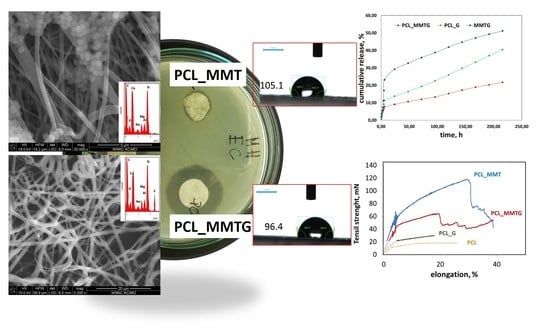
3. Results
3.1. X-ray Analysis of the Starting Montmorillonite Powders
3.2. Grain Size Distribution of Montmorillonite Powders
3.3. Morphology of the Starting Montmorillonite Powders
3.4. Characteristics of the Microstructure of Nanobiocomposite Materials
3.5. Assessment of the Wettability of Composite Materials
3.6. Mechanical Properties of Nanobiocomposite Materials
3.7. Thermal Studies of Nanobiocomposite Materials
3.8. Assessment of Antibacterial Properties and Kinetics of Gentamicin Sulfate Release from Nanobiocomposite Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasirci, V.; Vrana, E.; Zorlutuna, P.; Ndreu, A.; Yilgor, P.; Basmanav, F.B.; Aydin, E. Nanobiomaterials: A review of the existing science and technology, and new approaches. J. Biomater. Sci. Polym. Ed. 2006, 17, 1241–1268. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Ludueña, L.N.; Álvarez, V.; Vazquez, A. Processing and microstructure of PCL/clay nanocomposites. Mater. Sci. Eng. A 2007, 460–461, 121–129. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and barrier properties of poly(ɛ-caprolactone)-layered silicate nanocomposites. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1047–1057. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability Properties of Polymer−Layered-Silicate Nanocomposites. Polypropylene and Polystyrene Nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Gorrasi, G.; Tortora, M.; Vittoria, V.; Pollet, E.; Lepoittevin, B.; Alexandre, M.; Dubois, P. Vapor barrier properties of polycaprolactone montmorillonite nanocomposites: Effect of clay dispersion. Polymer 2003, 44, 2271–2279. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.-L.; Friedrich, K. Creep resistant polymeric nanocomposites. Polymer 2004, 45, 3481–3485. [Google Scholar] [CrossRef]
- Nosrati, H.; Khouy, R.A.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1–21. [Google Scholar] [CrossRef]
- Es-Haghi, A.; Mashreghi, M.; Bazaz, M.R.; Homayouni-Tabrizi, M.; Darroudi, M. Fabrication of biopolymer based nanocomposite wound dressing: Evaluation of wound healing properties and wound microbial load. IET Nanobiotechnol. 2017, 11, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Pogodina, N.V.; Cerclé, C.; Avérous, L.; Thomann, R.; Bouquey, M.; Muller, R. Processing and characterization of biodegradable polymer nanocomposites: Detection of dispersion state. Rheol. Acta 2008, 47, 543–553. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Drzal, L.T.; Misra, M. Nano-reinforcement of biobased polymers—The hope and reality. Polym. Mater. Sci. Eng. 2003, 88, 60–61. [Google Scholar]
- Pinnavaia, T.J.; Beall, G.W. Polymer—Clay Nanocomposites, Wiley Series in Polymer Science; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Das, P.; Malho, J.M.; Rahimi, K.; Schacher, F.H.; Wang, B.C.; Demco, D.E.; Walther, A. Nacre-mimetics with synthetic nanoclays up to ultrahigh aspect ratios. Nat. Commun. 2015, 6, 5967. [Google Scholar]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Noth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581. [Google Scholar] [CrossRef]
- Sinha, R.S.; Okamoto, K.; Okamoto, M. Structure-property relationship in biodegradable poly(butylene succinate)/layered silicate nanocomposites. Macromolecules 2003, 36, 2355–2367. [Google Scholar] [CrossRef]
- Rhim, J.-W. Effect of PLA lamination on performance characteristics of agar/κ-carrageenan/clay bio-nanocomposite film. Food Res. Int. 2013, 51, 714–722. [Google Scholar] [CrossRef]
- Umamaheswara Raoa, R.; Venkatanarayana, B.; Suman, K.N.S. Enhancement of Mechanical Properties of PLA/PCL (80/20) Blend by Reinforcing with MMT Nanoclay. Mater. Today Proc. 2019, 18, 85–97. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Camino, G. Nanocomposites of PLA and PCL based on montmorillonite and sepiolite. Mater. Sci. Eng. C 2009, 29, 1433–1441. [Google Scholar] [CrossRef]
- Bujok, S.; Peter, J.; Halecký, M.; Ecorchard, P.; Machálková, A.; Medeiros, G.S.; Hodan, J.; Pavlova, E.; Beneš, H. Sustainable microwave synthesis of biodegradable active packaging films based on polycaprolactone and layered ZnO nanoparticles. Polym. Degrad. Stab. 2021, 190, 109625. [Google Scholar] [CrossRef]
- Rostamian, M.; Kalaee, M.R.; Dehkordi, S.R.; Panahi-Sarmad, M.; Tirgar, M.; Goodarzi, V. Design and characterization of poly(glycerol-sebacate)-co-poly(caprolactone) (PGS-co-PCL) and its nanocomposites as novel biomaterials: The promising candidate for soft tissue engineering. Eur. Polym. J. 2020, 138, 109985. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Pierchała, M.K.; Tomas-Trybuś, A.; Szaraniec, B.; Karwot, J. The wettability, mechanical and antimicrobial properties of polylactide/montmorillonite nanocomposite films. Acta Bioeng. Biomech. 2017, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rapacz-Kmita, A. Montmorillonite as a drug carrier. Eng. Biomater. 2017, 139, 8–17. [Google Scholar]
- Albdiry, M.T.; Yousif, B.; Ku, H.; Lau, K.T. A critical review on the manufacturing processes in relation to the properties of nanoclay/polymer composites. J. Compos. Mater. 2012, 47, 1093–1115. [Google Scholar] [CrossRef]
- Nien, Y.-T.; Liao, Y.-H.; Liao, P.-C. Antibacterial activity of poloxamer-modified montmorillonite clay against E. coli. Mater. Lett. 2011, 65, 3092–3094. [Google Scholar] [CrossRef]
- Maryan, A.S.; Montazer, M. Natural and organo-montmorillonite as antibacterial nanoclays for cotton garment. J. Ind. Eng. Chem. 2015, 22, 164–170. [Google Scholar] [CrossRef]
- Magaña, S.; Quintana, P.; Aguilar, D.; Toledo, J.; Ángeles-Chávez, C.; Cortés, M.; León, L.; Freile-Pelegrin, Y.; López, T.; Sánchez, R.M.T. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A Chem. 2008, 281, 192–199. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Bućko, M.; Stodolak-Zych, E.; Mikołajczyk, M.; Dudek, P.; Trybus, M. Characterisation, in vitro release study, and antibacterial activity of montmorillonite-gentamicin complex material. Mater. Sci. Eng. C 2017, 70, 471–478. [Google Scholar] [CrossRef]
- He, H.; Yang, D.; Yuan, P.; Shen, W.; Frost, R.L. A novel organoclay with antibacterial activity prepared from montmorillonite and Chlorhexidini Acetas. J. Colloid Interface Sci. 2006, 297, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Ibarguren, C.; Naranjo, P.M.; Stötzel, C.; Audisio, M.C.; Sham, E.L.; Torres, E.M.F.; Müller, F.A. Adsorption of nisin on raw montmorillonite. Appl. Clay Sci. 2014, 90, 88–95. [Google Scholar] [CrossRef]
- Pantoustier, N.; Lepoittevin, B.; Alexandre, M.; Dubois, P.; Kubies, D.; Calberg, C.; Jérôme, R. Biodegradable polyester layered silicate nanocomposites based on poly(ϵ-caprolactone). Polym. Eng. Sci. 2002, 42, 1928–1937. [Google Scholar] [CrossRef]
- Di, Y.; Iannace, S.; Di Maio, E.; Nicolais, L. Nanocomposites by melt intercalation based on polycaprolactone and organoclay. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 670–678. [Google Scholar] [CrossRef]
- Paul, M.-A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/montmorillonite nanocomposites: Study of the hydrolytic degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Eckel, D.F.; Balogh, M.P.; Fasulo, P.D.; Rodgers, W.R. Assessing organo-clay dispersion in polymer nanocomposites. J. Appl. Polym. Sci. 2004, 93, 1110–1117. [Google Scholar] [CrossRef]
- Majdzadeh-Ardakani, K.; Lofgren, E.A.; Jabarin, S.A. Novel preparation method for improving the dispersion of ionic liquid-modified montmorillonite in poly(ethylene terephthalate). Polym. Compos. 2014, 37, 1259–1266. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Agubra, V.A.; Owuor, P.S.; Hosur, M.V. Influence of Nanoclay Dispersion Methods on the Mechanical Behavior of E-Glass/Epoxy Nanocomposites. Nanomaterials 2013, 3, 550–563. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Ray, S.S. The quantitative analysis of nano-clay dispersion in polymer nanocomposites by small angle X-ray scattering combined with electron microscopy. Polymer 2010, 51, 1437–1449. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Rivero, P.J.; Vicente, A.; Rodriguez, R.J. Electrospinning Technique as a Powerful Tool for the Design of Superhydrophobic Surfaces. In 21st Century Surface Science—A Handbook; InTech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Alarifi, I.; Alharbi, A.; Khan, W.S.; Swindle, A.L.; Asmatulu, R. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials 2015, 8, 7017–7031. [Google Scholar] [CrossRef] [Green Version]
- Leonés, A.; Mujica-Garcia, A.; Arrieta, M.P.; Salaris, V.; Lopez, D.; Kenny, J.M.; Peponi, L. Organic and Inorganic PCL-Based Electrospun Fibers. Polymer 2020, 12, 1325. [Google Scholar] [CrossRef]
- Yam, W.; Ismail, J.; Kammer, H.; Schmidt, H.; Kummerlöwe, C. Polymer blends of poly(ϵ-caprolactone) and poly(vinyl methyl ether)—Thermal properties and morphology. Polymer 1999, 40, 5545–5552. [Google Scholar] [CrossRef]
- Convention of United States Pharmacopeial, United States Pharmacopeia Content of Gentamicin Sulfate. 2011. Available online: https://extranet.who.int/nonstateactorsstatements/content/united-states-pharmacopeial-convention-5 (accessed on 9 November 2021).
- Chandrasekaran, A.R.; Jia, C.Y.; Theng, C.S.; Muniandy, T.; Muralidharan, S.; Dhanaraj, S.A. In vitro studies and evaluation of metformin marketed tablets-Malaysia. J. Appl. Pharm. Sci. 2011, 1, 214–217. [Google Scholar]
- Rapacz-Kmita, A.; Stodolak-Zych, E.; Ziąbka, M.; Różycka, A.; Dudek, M. Instrumental characterization of the smectite clay–gentamicin hybrids. Bull. Mater. Sci. 2015, 38, 1069–1078. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2016. [Google Scholar]
- Chang, J.-H.; An, Y.U.; Sur, G.S. Poly(lactic acid) nanocomposites with various organoclays. I. Thermomechanical properties, morphology, and gas permeability. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 94–103. [Google Scholar] [CrossRef]
- Feijoo, J.L.; Cabedo, L.; Lagaron, J.M.; Saura, J.J. Development of amorphous PLA-montmorillonite nanocomposites. J. Mater. Sci. 2005, 40, 1785–1788. [Google Scholar] [CrossRef]
- Hoidy, W.H.; Ahmad, M.B.; Al-Mulla, E.A.; Ibrahim, N.A. Preparation and Characterization of Polylactic Acid/Polycaprolactone Clay Nanocomposites. J. Appl. Sci. 2010, 10, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Reshmi, C.R.; Suja, P.S.; Manaf, O.; Sanu, P.P.; Sujith, A. Nanochitosan enriched poly ε-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Electrospun composite matrices of poly(ε-caprolactone)-montmorillonite made using tenside free Pickering emulsions. Mater. Sci. Eng. C 2016, 69, 685–691. [Google Scholar] [CrossRef]
- Pal, J.; Sharma, S.; Sanwaria, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Conducive 3D porous mesh of poly(ε-caprolactone) made via emulsion electrospinning. Polymer 2014, 55, 3970–3979. [Google Scholar] [CrossRef]
- Saeed, K.; Park, S.Y. Effect of nanoclay on the thermal, mechanical, and crystallization behavior of nanofiber webs of nylon-6. Polym. Compos. 2011, 33, 192–195. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Rong, J.; Nie, G.; Qiao, J.; Wang, H.; Wu, D.; Su, Z.; Niu, Z.; Huang, Y. Enhanced orientation of PEO polymer chains induced by nanoclays in electrospun PEO/clay composite nanofibers. Colloid Polym. Sci. 2013, 291, 1541–1546. [Google Scholar] [CrossRef]
- Sołtysiak, E.; Błażewicz, M. Influence of nanoparticles on physical and chemical surface material properties. Eng. Biomat. 2010, 92, 30–33. [Google Scholar]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Conte, A.A.; Sun, K.; Hu, X.; Beachley, V.Z. Effects of Fiber Density and Strain Rate on the Mechanical Properties of Electrospun Polycaprolactone Nanofiber Mats. Front. Chem. 2020, 8, 610. [Google Scholar] [CrossRef]
- Le, Q.P.; Uspenskaya, M.V.; Olekhnovich, R.O.; Baranov, M.A. The Mechanical Properties of PVC Nanofiber Mats Obtained by Electrospinning. Fibers 2021, 9, 2. [Google Scholar] [CrossRef]
- Wei, X.; Xia, Z.; Wong, S.C.; Baji, A. Modelling of mechanical properties of electrospun nanofibre network. Int. J. Exp. Comput. Biomech. 2009, 1, 45. [Google Scholar] [CrossRef]
- Yi, H.; Jia, F.; Zhao, Y.; Wang, W.; Song, S.; Li, H.; Liu, C. Surface wettability of montmorillonite (0 0 1) surface as affected by surface charge and exchangeable cations: A molecular dynamic study. Appl. Surf. Sci. 2018, 459, 148–154. [Google Scholar] [CrossRef]
- Ludueña, L.; Kenny, J.; Vázquez, A.; Álvarez, V. Effect of clay organic modifier on the final performance of PCL/clay nanocomposites. Mater. Sci. Eng. A 2011, 529, 215–223. [Google Scholar] [CrossRef]
- Merino, D.; Alvarez, V.A. Thermal degradation of poly (ε-caprolactone) nanocomposites with soy lecithin-modified bentonite fillers. Thermochim. Acta 2020, 689, 178638. [Google Scholar] [CrossRef]
- Khatoon, N.; Chu, M.Q.; Zhou, C.H. Nanoclay-based drug delivery systems and their therapeutic potentials. J. Mater. Chem. B 2020, 8, 7335–7351. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Scheibel, T. Copolymer/Clay Nanocomposites for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Walther, A.; Bjurhager, I.; Malho, J.-M.; Pere, J.; Ruokolainen, J.; Berglund, L.A.; Ikkala, O. Large-Area, Lightweight and Thick Biomimetic Composites with Superior Material Properties via Fast, Economic, and Green Pathways. Nano Lett. 2010, 10, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Lvov, Y.M. Halloysite clay nanotubes for tissue engineering. Nanomedicine 2016, 11, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Biswas, A.; Senapati, S.; Ray, B.; Rana, D.; Aswal, V.K.; Maiti, P. Novel shape memory behaviour in IPDI based polyurethanes: Influence of nanoparticle. Polymer 2017, 110, 95–104. [Google Scholar] [CrossRef]









| Materials | PCL | PCL_MMTG | PCL_G | PCL_MMT |
|---|---|---|---|---|
| Ultrasound homogenization, min/Hz | - | 3/60 | 1/60 | 1/60 |
| Chamber humidity, % | 20 | 25 | 21 | 21 |
| Chamber temperature, °C | 21 | 20 | 21 | 20 |
| Voltage, kV | 15 | 18 | 18 | 16 |
| Needle-collector distance, cm | 10 | 11 | 12 | 12 |
| Collector rotational speed, cm/min | 5 | |||
| The flow rate of the solution stream, mL/min | 1.2 | 1.8 | 1.0 | 1.5 |
| Powder | Main Peak Location, 2θ | Interlayer Distance d001, Å |
|---|---|---|
| MMT | 7.2 | 12.3 |
| MMTG | 6.3 | 13.9 |
| Material | Membrane Thickness, µm | Average Fiber Size, µm | Total Porosity of the Membrane, % | Water Absorption of the Fibrous Membrane, % |
|---|---|---|---|---|
| PCL | 206 ± 12 | 461 ± 75 | 92 | 59 |
| PCL_G | 178 ± 15 | 561 ± 67 | 89 | 66 |
| PCL_MMT | 210 ± 27 | 684 ± 135 | 86 | 62 |
| PCL_MMTG | 196 ± 24 | 591 ± 63 | 90 | 70 |
| Material | Tensile Strength MPa | Young’s Modulus MPa | Elongation at Break % |
|---|---|---|---|
| PCL | 0.029 | 0.54 | 23 |
| PCL_G | 0.037 | 0.56 | 19 |
| PCL_MMT | 0.112 | 1.22 | 31 |
| PCL_MMTG | 0.078 | 0.84 | 29 |
| Tg, °C | Tm, °C | Δf, J/mol | χ, % | |
|---|---|---|---|---|
| PCL | −53.7 | 62.8 | −71.2 | 45.3 |
| PCL_MMT | −63.7 | 64.7 | −87.1 | 55.6 |
| PCL_G | −61.2 | 63.9 | −88.2 | 56.2 |
| PCL_MMTG | −65.2 | 63.8 | −87.5 | 55.7 |
| Material | The Diameter of the Zone of Inhibition of Bacterial Growth, mm | |
|---|---|---|
| S. aureus | E. coli | |
| PCL | 15 | 14 |
| PCL_G | 25 | 24 |
| PLC_MMTG | 23 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stodolak-Zych, E.; Kurpanik, R.; Dzierzkowska, E.; Gajek, M.; Zych, Ł.; Gryń, K.; Rapacz-Kmita, A. Effects of Montmorillonite and Gentamicin Addition on the Properties of Electrospun Polycaprolactone Fibers. Materials 2021, 14, 6905. https://doi.org/10.3390/ma14226905
Stodolak-Zych E, Kurpanik R, Dzierzkowska E, Gajek M, Zych Ł, Gryń K, Rapacz-Kmita A. Effects of Montmorillonite and Gentamicin Addition on the Properties of Electrospun Polycaprolactone Fibers. Materials. 2021; 14(22):6905. https://doi.org/10.3390/ma14226905
Chicago/Turabian StyleStodolak-Zych, Ewa, Roksana Kurpanik, Ewa Dzierzkowska, Marcin Gajek, Łukasz Zych, Karol Gryń, and Alicja Rapacz-Kmita. 2021. "Effects of Montmorillonite and Gentamicin Addition on the Properties of Electrospun Polycaprolactone Fibers" Materials 14, no. 22: 6905. https://doi.org/10.3390/ma14226905