Drug Delivery with Polymeric Nanocarriers—Cellular Uptake Mechanisms
Abstract
:1. Introduction
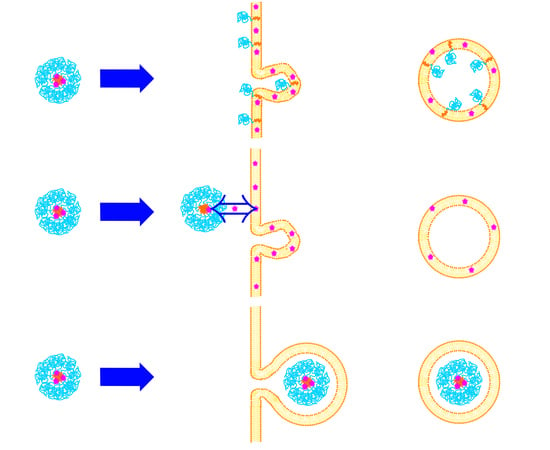
2. Endocytosis as the Main Uptake Mechanism in Cells
2.1. Phagocytosis
2.2. Pinocytosis
2.3. Elucidating Endocytic Pathways of Nanocarriers
3. Uptake Mechanisms of Polymeric Micelles
3.1. PEO-b-PCL Micelles
3.2. PEG-b-PLA
3.3. PEG-b-PLGA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Savić, R.; Luo, L.; Eisenberg, A.; Maysinger, D. Micellar Nanocontainers Distribute to Defined Cytoplasmic Organelles. Science 2003, 300, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiong, X.; Wan, J.; Xiao, L.; Gan, L.; Feng, Y.; Xu, H.; Yang, X. Cellular uptake and intracellular trafficking of PEG-b-PLA polymeric micelles. Biomaterials 2012, 33, 7233–7240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, X.; Liang, X.; Yang, Y.; Li, X.; Chen, H.; Huang, L.; Mei, L.; Feng, S.S. The chemotherapeutic potential of PEG-b-PLGA copolymer micelles that combine chloroquine as autophagy inhibitor and docetaxel as an anti-cancer drug. Biomaterials 2014, 35, 9144–9154. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric Micelles for Multi-Drug Delivery in Cancer. AAPS PharmSciTech 2014, 16, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Stern, T.; Kaner, I.; Laser Zer, N.; Shoval, H.; Dror, D.; Manevitch, Z.; Chai, L.; Brill-Karniely, Y.; Benny, O. Rigidity of polymer micelles affects interactions with tumor cells. J. Control. Release 2017, 257, 40–50. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Alfurhood, J.A.; Sun, H.; Kabb, C.P.; Tucker, B.S.; Matthews, J.H.; Luesch, H.; Sumerlin, B.S. Poly(N-(2-hydroxypropyl)methacrylamide)-valproic acid conjugates as block copolymer nanocarriers. Polym. Chem. 2017, 8, 4983–4987. [Google Scholar] [CrossRef] [PubMed]
- Luss, A.L.; Kulikov, P.P.; Romme, S.B.; Andersen, C.L.; Pennisi, C.P.; Docea, A.O.; Kuskov, A.N.; Velonia, K.; Mezhuev, Y.O.; Shtilman, M.I.; et al. Nanosized carriers based on amphiphilic poly-N-vinyl-2-pyrrolidone for intranuclear drug delivery. Nanomedicine 2018, 13, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Kulikov, P.P.; Kuskov, A.N.; Goryachaya, A.V.; Luss, A.N.; Shtil’man, M.I. Amphiphilic Poly-N-Vinyl-2-Pyrrolidone: Synthesis, Properties, Nanoparticles. Polym. Sci. Ser. D 2017, 10, 263–268. [Google Scholar] [CrossRef]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Li, X.; Qu, X.; Deng, Y.; Wang, Z.; He, C.; Zou, Y.; Jin, Y.; Liu, Y. Mechanisms of pH-Sensitivity and Cellular Internalization of PEOz-b-PLA Micelles with Varied Hydrophilic/Hydrophobic Ratios and Intracellular Trafficking Routes and Fate of the Copolymer. ACS Appl. Mater. Interfaces 2017, 9, 6916–6930. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Li, Y.; Yu, A.; Li, L.; Zhai, G. The development of stimuli-responsive polymeric micelles for effective delivery of chemotherapeutic agents. J. Drug Target. 2018, 26, 753–765. [Google Scholar] [CrossRef]
- Amjad, M.W.; Kesharwani, P.; Mohd Amin, M.C.I.; Iyer, A.K. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Prog. Polym. Sci. 2017, 64, 154–181. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.S.C.; Yadav, S.K.S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B. Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Zeng, X.; Liang, X.; Li, X.; Tao, W.; Chen, H.; Jiang, Y.; Mei, L.; Feng, S.S. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment. Biomaterials 2014, 35, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Callari, M.; De Souza, P.L.; Rawal, A.; Stenzel, M.H. The Effect of Drug Loading on Micelle Properties: Solid-State NMR as a Tool to Gain Structural Insight. Angew. Chem. Int. Ed. Engl. 2017, 56, 8441–8445. [Google Scholar] [CrossRef] [Green Version]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Rabinovitch, M. Professional and non-professional phagocytes: An introduction. Trends Cell Biol. 1995, 5, 85–87. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.F.; Porter, K.R. Yolk Protein Uptake in the Oocyte of the Mosquito Aedes Aegypti. L. J. Cell Biol. 1964, 20, 313–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Lorenz, A.; Dahlman, J.; Sahay, G. Challenges in carrier-mediated intracellular delivery: Moving beyond endosomal barriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 465–478. [Google Scholar] [CrossRef]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P.R. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [Green Version]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Zaki, N.M.; Tirelli, N. Gateways for the intracellular access of nanocarriers: A review of receptor-mediated endocytosis mechanisms and of strategies in receptor targeting. Expert Opin. Drug Deliv. 2010, 7, 895–913. [Google Scholar] [CrossRef]
- Pereira, P.; Pedrosa, S.S.; Wymant, J.M.; Sayers, E.; Correia, A.; Vilanova, M.; Jones, A.T.; Gama, F.M. SiRNA inhibition of endocytic pathways to characterize the cellular uptake mechanisms of folate-functionalized glycol chitosan nanogels. Mol. Pharm. 2015, 12, 1970–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Soraj, M.; He, L.; Peynshaert, K.; Cousaert, J.; Vercauteren, D.; Braeckmans, K.; De Smedt, S.C.; Jones, A.T. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J. Control. Release 2012, 161, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Iversen, T.G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis. Cell. Logist. 2013, 2, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, L.; Li, Y.; Wang, X.; Tu, P. Apically targeted oral micelles exhibit highly efficient intestinal uptake and oral absorption. Int. J. Nanomed. 2018, 13, 7997–8012. [Google Scholar] [CrossRef] [Green Version]
- Ghetler, Y.; Yavin, S.; Shalgi, R.; Arav, A. The effect of chilling on membrane lipid phase transition in human oocytes and zygotes. Hum. Reprod. 2005, 20, 3385–3389. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Kim, S.; Li, L.; Wang, S.; Park, K.; Cheng, J.-X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 6596–6601. [Google Scholar] [CrossRef] [Green Version]
- Mann, S.K.; Czuba, E.; Selby, L.I.; Such, G.K.; Johnston, A.P.R. Quantifying Nanoparticle Internalization Using a High Throughput Internalization Assay. Pharm. Res. 2016, 33, 2421–2432. [Google Scholar] [CrossRef]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The use of inhibitors to study endocytic pathways of gene carriers: Optimization and pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götte, M.; Sofeu Feugaing, D.D.; Kresse, H. Biglycan is internalized via a chlorpromazine-sensitive route. Cell. Mol. Biol. Lett. 2004, 9, 475–481. [Google Scholar] [PubMed]
- Wang, L.-H.; Rothberg, K.G.; Anderson, R.G.W. Mis-Assembly of Clathrin Lattices on Endosomes Reveals a Regulatory Switch for Coated Pit Formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I. Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful? In Exocytosis and Endocytosis; Ivanov, A.I., Ed.; Humana Press: Totowa, NJ, USA, 2008; ISBN 978-1-59745-178-9. [Google Scholar]
- Qaddoumi, M.G.; Gukasyan, H.J.; Lee, V.H.L.; Davda, J.; Labhasetwar, V.; Kim, K.-J. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: Role in PLGA nanoparticle endocytosis. Mol. Vis. 2003, 9, 559–568. [Google Scholar]
- Carpentier, J.-L.; Sawano, F.; Geiger, D.; Gorden, P.; Perrelet, A.; Orci, L. Potassium depletion and hypertonic medium reduce “non-coated” and clathrin-coated pit formation, as well as endocytosis through these two gates. J. Cell. Physiol. 1989, 138, 519–526. [Google Scholar] [CrossRef]
- Gibson, A.E.; Noel, R.J.; Herlihy, J.T.; Ward, W.F. Phenylarsine oxide inhibition of endocytosis: Effects on asialofetuin internalization. Am. J. Physiol. Physiol. 2017, 257, C182–C184. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.H.; Zheng, X.W.; Liu, Y.J.; Zhang, H.; Fang, L.; Zhang, Y.W.; Yang, C.; Islam, K.; Wang, C.; et al. Phenylarsine oxide (PAO) induces apoptosis in HepG2 cells: Via ROS-mediated mitochondria and ER-stress dependent signaling pathways. Metallomics 2017, 9, 1756–1764. [Google Scholar] [CrossRef]
- Altankov, G.; Grinnell, F. Depletion of intracellular potassium disrupts coated pits and reversibly inhibits cell polarization during fibroblast spreading. J. Cell Biol. 1993, 120, 1449–1459. [Google Scholar] [CrossRef]
- Preta, G.; Cronin, J.G.; Sheldon, I.M. Dynasore—Not just a dynamin inhibitor. Cell Commun. Signal. 2015, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- Kirchhausen, T.; Macia, E.; Pelish, H.E. Use of Dynasore, the Small Molecule Inhibitor of Dynamin, in the Regulation of Endocytosis. Methods Enzym. 2008, 438, 77–93. [Google Scholar]
- Pelkmans, L.; Püntener, D.; Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Joggerst, B.; Simons, K. Regulated internalization of caveolae. J. Cell Biol. 1994, 127, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Milhaud, J. Permeabilizing action of filipin III on model membranes through a filipin-phospholipid binding. BBA Biomembranes 1992, 1105, 307–318. [Google Scholar] [CrossRef]
- Song, X.; Li, R.; Deng, H.; Li, Y.; Cui, Y.; Zhang, H.; Dai, W.; He, B.; Zheng, Y.; Wang, X.; et al. Receptor mediated transcytosis in biological barrier: The influence of receptor character and their ligand density on the transmembrane pathway of active-targeting nanocarriers. Biomaterials 2018, 180, 78–90. [Google Scholar] [CrossRef]
- Sidaway, J.E.; Davidson, R.G.; McTaggart, F.; Orton, T.C.; Scott, R.C.; Smith, G.J.; Brunskill, N.J. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J. Am. Soc. Nephrol. 2004, 15, 2258–2265. [Google Scholar] [CrossRef] [Green Version]
- Loike, J.D.; Shabtai, D.Y.; Neuhut, R.; Malitzky, S.; Lu, E.; Husemann, J.; Goldberg, I.J.; Silverstein, S.C. Statin inhibition of Fc receptor-mediated phagocytosis by macrophages is modulated by cell activation and cholesterol. Arter. Thromb. Vasc. Biol. 2004, 24, 2051–2056. [Google Scholar] [CrossRef] [Green Version]
- Rodal, S.K.; Skretting, G.; Garred, Ø.; Vilhardt, F.; Van Deurs, B.; Sandvig, K. Extraction of Cholesterol with Methyl-b-Cyclodextrin Perturbs Formation of Clathrin-coated Endocytic Vesicles. 1999, 10, 961–974. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef]
- Hirama, T.; Fairn, G.D. Induction of spontaneous curvature and endocytosis: Unwanted consequences of cholesterol extraction using methyl-β-Cyclodextrin. Commun. Integr. Biol. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Hedin, U.; Thyberg, J. Receptor-mediated endocytosis of immunoglobulin-coated colloidal gold particles in cultured mouse peritoneal macrophages. Chloroquine and monensin inhibit transfer of the ligand from endocytic vesicles to lysosomes. Eur. J. Cell Biol. 1985, 39, 130–135. [Google Scholar]
- Veldhoen, S.; Laufer, S.D.; Trampe, A.; Restle, T. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: Quantitative analysis of uptake and biological effect. Nucleic Acids Res. 2006, 34, 6561–6573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, C.H.; Cho, M.K.; Shin, S.; Yoo, T.H.; Kim, H.M. Real-time monitoring of vesicle pH in an endocytic pathway using an EGF-conjugated two-photon probe. Chem. Commun. 2016, 52, 14007–14010. [Google Scholar] [CrossRef]
- Oishi, M.; Kataoka, K.; Nagasaki, Y. pH-responsive three-layered PEGylated polyplex micelle based on a lactosylated ABC triblock copolymer as a targetable and endosome-disruptive nonviral gene vector. Bioconjug. Chem. 2006, 17, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Uherek, C.; Fominaya, J.; Wels, W. A Modular DNA Carrier Protein Based on the Structure of Diphtheria Toxin Mediates Target Cell-specific Gene Delivery. J. Biol. Chem. 1998, 273, 8835–8841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, M.M.; Köping-Höggård, M.; Tømmeraas, K.; Vårum, K.M.; Christensen, B.E.; Strand, S.P.; Artursson, P. Targeted gene delivery with trisaccharide-substituted chitosan oligomers in vitro and after lung administration in vivo. J. Control. Release 2006, 115, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Tagawa, Y.; Yoshimori, T.; Moriyama, Y.; Masaki, R.; Tashiro, Y. Bafilomycin A1 Prevents Maturation of Autophagic Vacuoles by Inhibiting Fusion between Autophagosomes and Lysosomes in Rat Hepatoma Cell Line, H-4-II-E Cells. Cell Struct. Funct. 1998, 23, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Mauvezin, C.; Nagy, P.; Juhász, G.; Neufeld, T.P. Autophagosome-lysosome fusion is independent of V-ATPase-mediated acidification. Nat. Commun. 2015, 6, 7007. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.; van Galen, M.; Scherphof, G.L. Effects of Ammonium Chloride and Chloroquine on Endocytic Uptake of Liposomes by Kupffer Cells in Vitro. BBA Mol. Cell Res. 1984, 804, 58–67. [Google Scholar] [CrossRef]
- Browning, D.J. Hydroxychloroquine and Chloroquine Retinopathy; Springer: New York, NY, USA, 2014; ISBN 9781493905973. [Google Scholar]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, N.; Mathis, J.M.; Chen, M.; Pérez, R.L.; Siraj, N.; Magut, P.K.S.; McDonough, K.; Sahasrabudhe, G.; Warner, I.M. Endocytic Selective Toxicity of Rhodamine 6G nanoGUMBOS in Breast Cancer Cells. Mol. Pharm. 2018, 15, 3837–3845. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, L.M.; Roth, R.; Heuser, J.E.; Schmid, S.L. Actin Assembly Plays a Variable, but not Obligatory Role in Receptor-Mediated Endocytosis. Traffic 2000, 1, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.Y.; Wong, M.S.; Wang, Y. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: Preparation, in vitro evaluation, and cellular uptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Fenteany, G.; Zhu, S. Small-Molecule Inhibitors of Actin Dynamics and Cell Motility. Curr. Top. Med. Chem. 2003, 3, 593–616. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Schwab, B.; Thompson, N.C.; Elson, E.L. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2000, 114, 1025–1036. [Google Scholar]
- Yarmola, E.G.; Somasundaram, T.; Boring, T.A.; Spector, I.; Bubb, M.R. Actin-latrunculin a structure and function: Differential modulation of actin-binding protein function by latrunculin A. J. Biol. Chem. 2000, 275, 28120–28127. [Google Scholar] [CrossRef] [Green Version]
- Bubb, M.R.; Spector, I.; Bershadsky, A.D.; Korn, E.D. Swinholide A is a microfilament disrupting marine toxin that stabilizes actin dimers and severs actin filaments. J. Biol. Chem. 1995, 270, 3463–3466. [Google Scholar] [CrossRef] [Green Version]
- Corvera, S.; Czech, M.P. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 1998, 8, 442–446. [Google Scholar] [CrossRef]
- Jess, T.J.; Thomson, F.J.; Gould, G.W.; Belham, C.M.; Scott, P.H.; Plevin, R.J. Phosphatidylinositol 3’-kinase, but not p70 ribosomal S6 kinase, is involved in membrane protein recycling: Wortmannin inhibits glucose transport and downregulates cell-surface transferrin receptor numbers independently of any effect on fluid-phase endocy. Cell. Signal. 1996, 8, 297–304. [Google Scholar] [CrossRef]
- Araki, N.; Hamasaki, M.; Egami, Y.; Hatae, T. Effect of 3-methyladenine on the Fusion Process of Macropinosomes in EGF-stimulated A431 Cells. Cell Struct. Funct. 2006, 31, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Zhang, Y.; Liu, Y.; Chen, Q.; Fu, Y.; Liang, J.; Zhou, J.; Tang, X.; Liu, J.; Huo, M. The efficiency and mechanism of N-octyl-O, N-carboxymethyl chitosan-based micelles to enhance the oral absorption of silybin. Int. J. Pharm. 2018, 536, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Li, J. Formation, microstructure, biodistribution and absence of toxicity of polymeric micelles formed by N-octyl-N,O-carboxymethyl chitosan. Carbohydr. Polym. 2011, 83, 1959–1969. [Google Scholar] [CrossRef]
- Huo, M.; Fu, Y.; Liu, Y.; Chen, Q.; Mu, Y.; Zhou, J.; Li, L.; Xu, W.; Yin, T. N-mercapto acetyl-N′-octyl-O, N″-glycol chitosan as an efficiency oral delivery system of paclitaxel. Carbohydr. Polym. 2018, 181, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Yu, D.; Wu, Y.; Li, J.; Li, H. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int. J. Pharm. 2010, 394, 162–173. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.F.; Quan, L.H.; Liu, C.Y.; Liu, X.M.; Ehrhardt, C.; Liao, Y.H. Pulmonary delivered polymeric micelles—Pharmacokinetic evaluation and biodistribution studies. Eur. J. Pharm. Biopharm. 2014, 88, 1064–1075. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.F.; Liu, C.Y.; Ehrhardt, C.; Liao, Y.H. In vitro uptake and transport studies of PEG-PLGA polymeric micelles in respiratory epithelial cells. Eur. J. Pharm. Biopharm. 2017, 114, 29–37. [Google Scholar] [CrossRef]
- Song, Q.; Wang, X.; Hu, Q.; Huang, M.; Yao, L.; Qi, H.; Qiu, Y.; Jiang, X.; Chen, J.; Chen, H.; et al. Cellular internalization pathway and transcellular transport of pegylated polyester nanoparticles in Caco-2 cells. Int. J. Pharm. 2013, 445, 58–68. [Google Scholar] [CrossRef]
- Mahmud, A.; Lavasanifar, A. The effect of block copolymer structure on the internalization of polymeric micelles by human breast cancer cells. Colloids Surf. B Biointerfaces 2005, 45, 82–89. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.H.; Zhang, J.; Zhang, G.; Gan, Z.H. Effect of surface charge of polymeric micelles on in vitro cellular uptake. Chin. J. Polym. Sci. (Engl. Ed.) 2013, 31, 1299–1309. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C.; Szewczyk, A. Cellular Distribution of Nonionic Micelles. Science 2004, 303, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Chang, T.; Gosain, P.; Stenzel, M.H.; Lord, M.S. Drug-loading of poly(ethylene glycol methyl ether methacrylate) (PEGMEMA)-based micelles and mechanisms of uptake in colon carcinoma cells. Colloids Surf. B Biointerfaces 2016, 144, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiong, X.; Sun, X.; Zhu, Y.; Yang, H.; Chen, H.; Gan, L.; Xu, H.; Yang, X. Role of cellular uptake in the reversal of multidrug resistance by PEG-b-PLA polymeric micelles. Biomaterials 2011, 32, 5148–5157. [Google Scholar] [CrossRef]
- Till, U.; Gibot, L.; Mingotaud, A.F.; Ehrhart, J.; Wasungu, L.; Mingotaud, C.; Souchard, J.P.; Poinso, A.; Rols, M.P.; Violleau, F.; et al. Drug release by direct jump from poly(ethylene-glycol-b-ϵ-caprolactone) nano-vector to cell membrane. Molecules 2016, 21, 1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Tam, J.; Maysinger, D.; Eisenberg, A. Cellular internalization of poly(ethylene oxide)-b-poly(ε-caprolactone) diblock copolymer micelles. Bioconjug. Chem. 2002, 13, 1259–1265. [Google Scholar] [CrossRef]
- Allen, C.; Yu, Y.; Eisenberg, A.; Maysinger, D. Cellular internalization of PCL20-b-PEO44block copolymer micelles. Biochim. Biophys. Acta Biomembr. 1999, 1421, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Kerdous, R.; Sureau, F.; Bour, A.; Bonneau, S. Release kinetics of an amphiphilic photosensitizer by block-polymer nanoparticles. Int. J. Pharm. 2015, 495, 750–760. [Google Scholar] [CrossRef]
- Kuzelova, K.; Brault, D. Kinetic and equilibrium studies of porphyrin interactions with unilamellar lipidic vesicles. Biochemistry 1994, 33, 9447–9459. [Google Scholar] [CrossRef]
- Aldwinckle, T.J.; Ahkong, Q.F.; Bangham, A.D.; Fisher, D.; Lucy, J.A. Effects of poly(ethylene glycol) on liposomes and erythrocytes: Permeability changes and membrane fusion. Biochim. Biophys. Acta Biomembr. 1982, 689, 548–560. [Google Scholar] [CrossRef]
- Maysinger, D.; Berezovska, O.; Savic, R.; Lim Soo, P.; Eisenberg, A. Block copolymers modify the internalization of micelle-incorporated probes into neural cells. Biochim. Biophys. Acta Mol. Cell Res. 2001, 1539, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Savić, R.; Azzam, T.; Eisenberg, A.; Maysinger, D. Assessment of the integrity of poly(caprolactone)-b-poly(ethylene oxide) micelles under biological conditions: A fluorogenic-based approach. Langmuir 2006, 22, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Yasugi, K.; Nagasaki, Y.; Kato, M.; Kataoka, K. Preparation and characterization of polymer micelles from poly(ethylene glycol)-poly(D,L-lactide) block copolymers as potential drug carrier. J. Control. Release 1999, 62, 89–100. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, L.; Moingeon, F.; Gauthier, M.; Yang, G. PH-Responsive Poly(Ethylene Glycol)-block-Polylactide Micelles for Tumor-Targeted Drug Delivery. Biomacromolecules 2017, 18, 2711–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Cheng, J.-X.; Kim, S.; Low, P.S.; Wang, H.; Chen, H.; Park, K. Fast Release of Lipophilic Agents from Circulating PEG-PDLLA Micelles Revealed by in Vivo Förster Resonance Energy Transfer Imaging. Langmuir 2008, 24, 5213–5217. [Google Scholar]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef]
- Babos, G.; Biró, E.; Meiczinger, M.; Feczkó, T. Dual Drug Delivery of Sorafenib and Doxorubicin from PLGA and PEG-PLGA Polymeric Nanoparticles. Polymers 2018, 10, 895. [Google Scholar] [CrossRef] [Green Version]
- Rompicharla, S.V.K.; Trivedi, P.; Kumari, P.; Muddineti, O.S.; Theegalapalli, S.; Ghosh, B.; Biswas, S. Evaluation of Anti-Tumor Efficacy of Vorinostat Encapsulated Self-Assembled Polymeric Micelles in Solid Tumors. AAPS PharmSciTech 2018, 19, 3141–3151. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Huang, H.; Huang, J.; Deng, A.; Zou, P.; Tan, X. Bone-targeted delivery of simvastatin-loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment. Drug Deliv. Transl. Res. 2018, 8, 1090–1102. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Ahmed, O.A.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shen, X.; Sun, X.; Peng, Y.; Li, R.; Yun, P.; Li, C.; Liu, L.; Su, F.; Li, S. Biocompatibility evaluation of self-assembled micelles prepared from poly(lactide-co-glycolide)-poly(ethylene glycol) diblock copolymers. Polym. Adv. Technol. 2018, 29, 205–215. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA-PEG block copolymer. J. Control. Release 2001, 70, 63–70. [Google Scholar] [CrossRef]







| Agent | Mechanism Affected 1 | Effect | Limitation | Ref. |
|---|---|---|---|---|
| Low temp (4 degrees) | All energy dependent processes | Slows down/inhibits all energy dependent processes | Low temperature may influence fluidity of cell membrane | [47,48] |
| Sodium azide | All energy dependent processes | Inhibits respiratory system of cells | Toxic at higher concentrations | [49,50] |
| Chlorpromazine | CME | Translocates clathrin and AP2 from the cell surface to intracellular endosomes | Not efficient in all cell lines, might interfere with the biogenesis of intracellular vesicles | [51,52,53] [54] (pp. 19–20) |
| Cytosol acidification | CME | Inhibits the budding-off of clathrin- coated pits from the membrane | Interferes with macropinocytosis and the actin cytoskeleton | [54] (p. 19) |
| Hypertonic sucrose | CME | Removes plasma membrane-associated clathrin lattices | Nonspecific, interferes with fluid phase macropinocytosis | [54] (pp. 17–18) [55,56] |
| Monodansylcadaverine | CME | Stabilizes clathrin-coated pits | Induces global changes in actin dynamics | [54] (p. 20) |
| Phenylarsine oxide | CME | Mechanisms unknown, possibly a tyrosine phosphate inhibitor | Also inhibits micropinocytosis and is toxic at higher concentrations | [57,58] |
| Potassium depletion | CME | Removes plasma membrane-associated clathrin lattices | Nonspecific; affects actin cytoskeleton | [54] (p. 18) [59] |
| Dynasore | CME, CvME | Inhibitor of dynamin (small GTPase) | Has other off-target effects, including inhibition of membrane ruffling | [60,61] |
| Genistein | CvME | Inhibitor of several tyrosine kinases, causes disruption of the actin network | Affects several uptake processes | [62,63] |
| Okadaic acid | CvME | Phosphatase inhibitor, stimulates trafficking and internalization of caveolae | Nonspecific, off-target effects | [64] |
| Cholesterol inhibitors | ||||
| Filipin | CvME, Lipid raft | Binds to cholesterol in the membrane | Unstable and toxic, cholesterol influences other endocytic pathways besides CvME | [54] (pp. 23–24) [65,66] |
| Statins | CvME, Lipid raft | Lowering of cholesterol formation by inhibiting the enzyme 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase | Nonspecific, many off-target effects | [54] (p. 22) [67,68] |
| Methyl-β-cyclodextrin | CvME, Lipid raft | Removes cholesterol out of the plasma membrane by forming soluble inclusion complexes with cholesterol | Nonspecific, interferes with fluid phase endocytosis and CME, might induce membrane curvature | [54] (pp. 22–23) [69,70] |
| Nystatin | CvME, Lipid raft | Binds to cholesterol in the membrane | Toxic | [54] (pp. 23–24) |
| (Endosome) acidification inhibitors | ||||
| Monensin | Prevents acidification of endosomes | Acts as an ionophor, thereby inhibiting the acidification of endosomes | [71,72,73] | |
| Nigericin | Prevents acidification of endosomes | Acts as an ionophor, thereby inhibiting the acidification of endosomes | [74,75] | |
| Bafilomycin A1 | Prevents acidification of endosomes | Inhibits the vacuolar ATPase endosomal proton pump. | Prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes. Potentially inhibits Ca2+ pump SERCA | [76,77,78] |
| Chloroquine | Prevents acidification of endosomes | Increases pH of acidic vesicles (e.g., lysosomes), possibly inhibits some lysosomal hydrolases | Affects many other cellular processes | [79,80] (pp. 49–54) |
| Amiloride | Macropinocytosis | Inhibits macropinocytosis by lowering submembranous pH (cytosolic pH close to the membrane) and prevents Rac1 and Cdc42 signaling. | [81,82,83] | |
| F-actin inhibitors | ||||
| Cytochalasin D | Macropinocytosis | Inhibits actin polymerization and may thus lead to actin filament disassembly | Nonspecific, may affect other endocytic processes | [54] (p. 26) [84,85] |
| Jasplakinolide | Macropinocytosis | Stabilizes actin and promotes actin assembly | Various effects depending on cell line and assay conditions | [84,86] |
| Latrunculin | Macropinocytosis | Sequesters actin monomers, blocks actin polymerization and may thus lead to actin filament disassembly | Not necessarily efficient in adherent cells | [54] (p. 26) [87,88] |
| Swinholide A | Macropinocytosis | Has F-actin severing activity | Nonspecific, may affect other endocytic processes | [86,89] |
| Phosphoinositide 3-kinase inhibitors | ||||
| LY294002 | Macropinocytosis | Inhibits phosphatidylinositol 3-kinase class I and III | Nonspecific, also affects CME and CvME | [54] (pp. 26–27) [90,91] |
| Wortmannin | Macropinocytosis | Inhibits phosphatidylinositol 3-kinase class I and III | Nonspecific, also affects CME and CvME | [54] (pp. 26–27) [90,91] |
| 3-methyladenine | Macropinocytosis | Inhibits phosphatidylinositol 3-kinase class III | Nonspecific, also affects CME and CvME | [54] (pp. 26–27) [92] |
| Material 1,2 | Uptake Mechanism(s) 1 | Cell type 3 | Drug 1 | Comments | Ref. |
|---|---|---|---|---|---|
| Mixed micelles: TPGS2K, HS15, F127 | Energy dependent CME CvME | Caco-2 | Curcumin DOX | Only analyzed uptake of drug | [93] |
| OCC | CME CvME | Caco-2 | Silybin Rhodamine-123 | Only analyzed uptake of drug | [94,95] |
| OGC SH-OGC | CME CvME | Caco-2 | Paclitaxel Rhodamine-123 | Only analyzed uptake of drug | [96,97] |
| P(PEGMEMA)75u-b-PMMA80u | Clathrin and caveolae independent CME CvME | WiDr | DOX NileRed | 80% of the uptake was via a different, undefined uptake mechanism | [106] |
| PEG2000-b-PLGA5000 | Energy dependent CME | Calu-3 NCI-H441 | NileRed Curcumin acetate | Only analyzed uptake of drug | [98,99] |
| PEG3000/2000/5000-PLA40000 PEG2000/5000-PLGA40000 | Energy dependent Lipid raft mediated | Caco-2 | Curcumin Coumarin 6 | Only analyzed uptake of drug | [100] |
| PEG5000-b-PLA5000 | Direct drug transfer to cell membrane Energy dependent Caveolae/lipid raft-mediated endocytosis | A2780 | Paclitaxel NileRed FRET, DAF/NileRed | [5,107] | |
| PEG-b-PLGA | CvME | MCF-7 | DTX, 3-MA, CQ Coumarin 6 | Only analyzed uptake of drug | [6] |
| PEO2000/5000/13000-b-PCL5000 PEO5000-b-PCL13000/24000 | CME | MCF-7 | DiIC | PEO5000-b-PCL13000 showed fastest uptake, only analyzed drug uptake | [101] |
| PEO2000-b-PCL2600/2800 PEO5000-b-PCL4000 | Direct drug transfer to cell membrane | HCT-116 | Pheo Conjugated Fluorescein | [108] | |
| PEO45u-b-PCL23u | Energy dependent CME | P19 | Conjugated Rhodamine | [3,109] | |
| PEO44u-b-PCL20u | Temperature, pH and energy dependent | PC12 | DiIC | Only analyzed uptake of drug | [110] |
| PEO5000-b-PCL2000 PEO5000-b-PDLLA5000 | Direct drug transfer to cell membrane | KB | DiIC/DiOC Conjugated Fluorescein | [49] | |
| PEO5000-b-PCL4000 | Direct drug transfer to cell membrane | MCF-7 | Pheo DiIC/DiOC | Micelle uptake is slow (>4 hr), while release of drug is fast | [111] |
| PEOz6000-b-PLA1100/2200/3900/8500/10000/13700 PEOz2600/3300/4500/5600/6700/8900-b-PLA4000 | Energy dependent Cholesterol dependent Caveolae/lipid raft-mediated endocytosis | MCF-7 | Paclitaxel Conjugated DEC | PEOz/PLA ratio of 1.7-2.0 for optimal uptake | [17] |
| Val-TPGS Phe-TPGS | Energy dependent CvME CME Macropinocytosis | Caco-2 | Curcumin Coumarin 6 | Enhanced transport across intestinal epithelial barrier, Only analyzed uptake of drug | [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelemans, L.C.; Gurevich, L. Drug Delivery with Polymeric Nanocarriers—Cellular Uptake Mechanisms. Materials 2020, 13, 366. https://doi.org/10.3390/ma13020366
Nelemans LC, Gurevich L. Drug Delivery with Polymeric Nanocarriers—Cellular Uptake Mechanisms. Materials. 2020; 13(2):366. https://doi.org/10.3390/ma13020366
Chicago/Turabian StyleNelemans, Levi Collin, and Leonid Gurevich. 2020. "Drug Delivery with Polymeric Nanocarriers—Cellular Uptake Mechanisms" Materials 13, no. 2: 366. https://doi.org/10.3390/ma13020366