Characterization of Base Oil and Additive Oxidation Products from Formulated Lubricant by Ultra-High Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. FTICR MS Analyses
2.3. Data Processing
3. Results and Discussion
3.1. Atmospheric Pressure Chemical Ionization Analysis
3.2. Electrospray Ionization Analysis
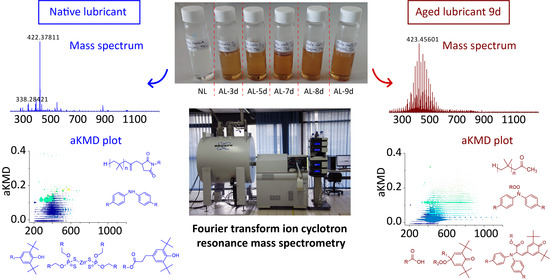
3.3. Native Lubricant Characterizations
3.4. Characterization of Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dev Srivyas, P.; Charoo, M.S. Effect of Lubricants Additive: Use and Benefit. Mater. Today Proc. 2019, 18, 4773–4781. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gondo, S. Friction and Wear Characteristics of Molybdenum Dithiocarbamate and Molybdenum Dithiophosphate. Tribol. Trans. 1989, 32, 251–257. [Google Scholar] [CrossRef]
- Bovington, C.H. Friction, wear and the role of additives in their control. In Chemistry and Technology of Lubricants; Mortier, R.M., Orszulik, S.T., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 320–348. [Google Scholar]
- Barnes, A.M.; Bartle, K.D.; Thibon, V.R.A. A review of zinc dialkyldithiophosphates (ZDDPS): Characterisation and role in the lubricating oil. Tribol. Int. 2001, 34, 389–395. [Google Scholar] [CrossRef]
- Tang, H.-Z.; Jao, T.-C. Dispersant Additives. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.-W., Eds.; Springer US: Boston, MA, USA, 2013; pp. 771–781. [Google Scholar]
- Rudnick, L.R. Lubricant Additives, 2nd ed.; Rudnick, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hsu, S.M.; Ku, C.K.; Pei, P.T. Oxidative Degradation Mechanisms of Lubricants. In Aspects of Lubricant Oxidation: A Symposium; ASTM International, Ed.; ASTM International: West Conshohocken, PA, USA, 1986; pp. 27–48. [Google Scholar]
- Shah, R.; Rizvi, S.Q.A.; Migdal, C.; DiNicola, K. Oxidation of Lubricants and Fuels. In Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing, 2nd ed.; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Murray, D.W.; MacDonald, J.M.; White, A.M.; Wright, P.G. SP 15 A New Concept of Lubricant Base Oil Quality. In Proceedings of the 11th World Petroleum Congress, London, UK, 28 August 1983. [Google Scholar]
- Aguilar, G.; Mazzamaro, G.; Rasberger, M. Oxidative Degradation and Stabilisation of Mineral Oil-Based Lubricants. In Chemistry and Technology of Lubricants; Mortier, R.M., Fox, M.F., Orszulik, S.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 107–152. [Google Scholar]
- El-Naggar, A.Y.; El-Adly, R.A.; Altalhi, T.A.; Alhadhrami, A.; Modather, F.; Ebiad, M.A.; Salem, A. Oxidation stability of lubricating base oils. Pet. Sci. Technol. 2018, 36, 179–185. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Perez, J.M.; Singh, I.D.; Tyagi, O.S. Spectroscopic Studies of Oxidative Degradation of Base Oils. Energy Fuels 1998, 12, 1369–1374. [Google Scholar] [CrossRef]
- Wilson, R.W.; Lyon, S.B. 2.26—Corrosion in Lubricants/Fuels*. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Oxford, UK, 2010; pp. 1299–1307. [Google Scholar]
- Klimov, A.K.; Baranov, V.A. Changes in viscosity of liquid lubricants in kinetic oxidation. Chem. Technol. Fuels Oils 1991, 27, 613–616. [Google Scholar] [CrossRef]
- Obiols, J. Lubricant Oxidation Monitoring Using FTIR Analysis—Application to the Development of a Laboratory Bulk Oxidation Test and to In-Service Oil Evaluation. SAE/SAE International Spring Fuels and Lubricants Meeting. May 2003, 112, 1903–1914. [Google Scholar]
- Lavison-Bompard, G.; Bertoncini, F.; Thiebaut, D.; Beziau, J.F.; Carraze, B.; Valette, P.; Duteurtre, X. Hypernated supercritical fluid chromatography: Potential application for car lubricant analysis. J. Chromatogr. A 2012, 1270, 318–323. [Google Scholar] [CrossRef]
- Snyder, S.R.; Wesdemiotis, C. Elucidation of Low Molecular Weight Polymers in Vehicular Engine Deposits by Multidimensional Mass Spectrometry. Energy Fuels 2020, 35, 1691–1700. [Google Scholar] [CrossRef]
- Dytkiewitz, E.; Morlock, G.E. Analytical strategy for rapid identification and quantification of lubricant additives in mineral oil by high-performance thin-layer chromatography with UV absorption and fluorescence detection combined with mass spectrometry and infrared spectroscopy. J. AOAC Int. 2008, 91, 1237–1243. [Google Scholar]
- Barrere, C.; Hubert-Roux, M.; Afonso, C.; Racaud, A. Rapid analysis of lubricants by atmospheric solid analysis probe-ion mobility mass spectrometry. J. Mass Spectrom. JMS 2014, 49, 709–715. [Google Scholar] [CrossRef]
- Becchi, M.; Perret, F.; Carraze, B.; Beziau, J.F.; Michel, J.P. Structural determination of zinc dithiophosphates in lubricating oils by gas chromatography-mass spectrometry with electron impact and electron-capture negative ion chemical ionization. J. Chromatogr. A 2001, 905, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Kassler, A.; Pittenauer, E.; Doerr, N.; Allmaier, G. Electrospray ionization and atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry of antioxidants applied in lubricants. Rapid Commun. Mass Spectrom. 2009, 23, 3917–3927. [Google Scholar] [CrossRef]
- Kassler, A.; Pittenauer, E.; Doerr, N.; Allmaier, G. Ultrahigh-performance liquid chromatography/electrospray ionization linear ion trap Orbitrap mass spectrometry of antioxidants (amines and phenols) applied in lubricant engineering. Rapid Commun. Mass Spectrom. 2014, 28, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Ramopoulou, L.; Widder, L.; Brenner, J.; Ristic, A.; Allmaier, G. Atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry of engine oil additive components. Rapid Commun. Mass Spectrom. 2022, 36, e9271. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Rodgers, R.P. Petroleomics: Chemistry of the underworld. Proc. Natl. Acad. Sci. USA 2008, 105, 18090. [Google Scholar] [CrossRef]
- Gutiérrez Sama, S.; Farenc, M.; Barrère-Mangote, C.; Lobinski, R.; Afonso, C.; Bouyssière, B.; Giusti, P. Molecular Fingerprints and Speciation of Crude Oils and Heavy Fractions Revealed by Molecular and Elemental Mass Spectrometry: Keystone between Petroleomics, Metallopetroleomics, and Petrointeractomics. Energy Fuels 2018, 32, 4593–4605. [Google Scholar] [CrossRef]
- Ruddy, B.M.; Blakney, G.T.; Rodgers, R.P.; Hendrickson, C.L.; Marshall, A.G. Elemental composition validation from stored waveform inverse Fourier transform (SWIFT) isolation FT-ICR MS isotopic fine structure. J. Am. Soc. Mass Spectrom. 2013, 24, 1608–1611. [Google Scholar] [CrossRef]
- Maillard, J.F.; Mase, C.; Serve, O.; Vezin, H.; Afonso, C.; Giusti, P.; Mangote, C. Characterization of copper-containing porphyrins in an insoluble fraction of a bio-crude obtained from hydrothermal liquefaction of wild microalgae. Algal Res. 2023, 73, 103166. [Google Scholar] [CrossRef]
- Kim, E.-K.; No, M.-H.; Koh, J.-S.; Kim, S. Compositional Characterization of Petroleum Heavy Oils Generated from Vacuum Distillation and Catalytic Cracking by Positive-mode APPI FT-ICR Mass Spectrometry. Mass Spectrom. Lett. 2011, 2, 41–44. [Google Scholar] [CrossRef]
- Hourani, N.; Muller, H.; Adam, F.M.; Panda, S.K.; Witt, M.; Al-Hajji, A.A.; Sarathy, S.M. Structural Level Characterization of Base Oils Using Advanced Analytical Techniques. Energy Fuels 2015, 29, 2962–2970. [Google Scholar] [CrossRef]
- Fouquet, T.; Sato, H. Improving the Resolution of Kendrick Mass Defect Analysis for Polymer Ions with Fractional Base Units. Mass Spectrom. (Tokyo Jpn.) 2017, 6, A0055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix-Andrivet, O.; Moualdi, S.; Hubert-Roux, M.; Loutelier Bourhis, C.; Mendes Siqueira, A.L.; Afonso, C. Molecular Characterization of Formulated Lubricants and Additive Packages Using Kendrick Mass Defect Determined by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2022, 33, 1194–1203. [Google Scholar] [CrossRef]
- Babić, N.; Pondaven, S.; Vezin, H. EPR Spin-Trapping Study of Free Radical Intermediates in Polyalphaolefin Base Oil Autoxidation. Polym. Degrad. Stab. 2021, 192, 109687. [Google Scholar] [CrossRef]
- Maillard, J.; Ferey, J.; Rüger, C.P.; Schmitz-Afonso, I.; Bekri, S.; Gautier, T.; Carrasco, N.; Afonso, C.; Tebani, A. Optimization of ion trajectories in a dynamically harmonized Fourier-transform ion cyclotron resonance cell using a design of experiments strategy. Rapid Commun. Mass Spectrom. 2020, 34, e8659. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics. Part 1: Asphaltenes Are Composed of Abundant Island and Archipelago Structural Motifs. Energy Fuels 2017, 31, 13509–13518. [Google Scholar] [CrossRef]
- Rüger, C.P.; Neumann, A.; Sklorz, M.; Schwemer, T.; Zimmermann, R. Thermal Analysis Coupled to Ultrahigh Resolution Mass Spectrometry with Collision Induced Dissociation for Complex Petroleum Samples: Heavy Oil Composition and Asphaltene Precipitation Effects. Energy Fuels 2017, 31, 13144–13158. [Google Scholar] [CrossRef]
- Maillard, J.; Schmitz-Afonso, I.; Gautier, T.; Afonso, C.; Carrasco, N. Suggested plausible structures for Titan’s haze analogs using tandem mass spectrometry. Icarus 2021, 358, 114181. [Google Scholar] [CrossRef]
- Shukla, A.K.; Futrell, J.H. Tandem mass spectrometry: Dissociation of ions by collisional activation. J. Mass Spectrom. JMS 2000, 35, 1069–1090. [Google Scholar] [CrossRef]
- Sueur, M.; Rüger, C.P.; Maillard, J.F.; Lavanant, H.; Zimmermann, R.; Afonso, C. Selective characterization of petroporphyrins in shipping fuels and their corresponding emissions using electron-transfer matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Fuel 2023, 332, 126283. [Google Scholar] [CrossRef]
- Rowland, R.; Dong, J.; Migdal, C. Antioxidants; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–36. [Google Scholar]
- Graham, J.; Spikes, H.; Korcek, S. The Friction Reducing Properties of Molybdenum Dialkyldithiocarbamate Additives: Part I—Factors Influencing Friction Reduction. Tribol. Trans. 2001, 44, 626–636. [Google Scholar] [CrossRef]
- Fouquet, T.N.J.; Cody, R.B.; Ozeki, Y.; Kitagawa, S.; Ohtani, H.; Sato, H. On the Kendrick Mass Defect Plots of Multiply Charged Polymer Ions: Splits, Misalignments, and How to Correct Them. J. Am. Soc. Mass Spectrom. 2018, 29, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, T. Flame Retardant Compound, Method of Making the Same, Resin Composition and Article Made Therefrom. U.S. Patent US10774266B2, 15 September 2020. [Google Scholar]
- Fujita, H.; Spikes, H.A. Study of Zinc Dialkyldithiophosphate Antiwear Film Formation and Removal Processes, Part II: Kinetic Model. Tribol. Trans. 2005, 48, 567–575. [Google Scholar] [CrossRef]
- Kiw, Y.M.; Schaeffer, P.; Adam, P.; Thiébaut, B.; Boyer, C.; Papin, G. Ligand exchange processes between molybdenum and zinc additives in lubricants: Evidence from NMR (1H, 13C, 31P) and HPLC-MS analysis. RSC Adv. 2020, 10, 37962–37973. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Dratva, A.; Spikes, H.A. Friction and Wear Behavior of Zinc Dialkyldithiophosphate Additive. Tribol. Trans. TRIBOL TRANS 2000, 43, 469–479. [Google Scholar] [CrossRef]
- Bae, W.-S.; Kovsky, J.R.; Tabor, R. Sustainable Base Oils for Lubricants. U.S. Patent US10336958B2, 2 July 2019. [Google Scholar]
- Ayabe, T.; Usui, T.; Yokomori, M. Light stabilizer composition and resin composition containing same. U.S. Patent US20160237241A1, 10 November 2014. [Google Scholar]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef]
- Mortier, R.; Fox, M.; Orszulik, S. Chemistry and Technology of Lubricants; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.B.; Coy, R.C. The Chemistry of the Thermal Degradation of Zinc Dialkyldithiophosphate Additives. A S L E Trans. 1981, 24, 91–97. [Google Scholar] [CrossRef]
- Ruffell, J.E.; Farmer, T.J.; Macquarrie, D.J.; Stark, M.S. The Autoxidation of Alkenyl Succinimides—Mimics for Polyisobutenyl Succinimide Dispersants. Ind. Eng. Chem. Res. 2019, 58, 19649–19660. [Google Scholar] [CrossRef]
- Gonon, L.; Troquet, M.; Fanton, E.; Gardette, J.-L. Thermo and photo-oxidation of polyisobutylene—II. Influence of the temperature. Polym. Degrad. Stab. 1998, 62, 541–549. [Google Scholar] [CrossRef]
- De Feo, M.; Minfray, C.; De Barros Bouchet, M.I.; Thiebaut, B.; Martin, J.M. MoDTC friction modifier additive degradation: Correlation between tribological performance and chemical changes. RSC Adv. 2015, 5, 93786–93796. [Google Scholar] [CrossRef]







| Samples | Aging DURATION (Day) |
|---|---|
| NL (native lubricant) | 0 |
| AL-3d (aged lubricant) | 3 |
| AL-5d (aged lubricant) | 5 |
| AL-7d (aged lubricant) | 7 |
| AL-8d (aged lubricant) | 8 |
| AL-9d (aged lubricant) | 9 |
| APCI(+) | ESI(−) | ||
|---|---|---|---|
| Source | Flow (µL h−1) | 600 | 600 |
| Nebulizer gas pressure (bar) | 2.0 | 1.0 | |
| Drying flow (L min−1) | 2.0 | 4.0 | |
| Drying temperature (°C) | 200 | 200 | |
| Vaporization temperature (°C) | 300 | - | |
| Corona needle (knA) | 4.0 | - | |
| Ion transfer | Capillary voltage (kV) | −4.5 | 3.9 |
| Quadrupole m/z | 150 | 150 | |
| Time of flight (ms) | 0.7 | 0.8 | |
| Acquisition | m/z range | 202.7–1300 | 147–1000 |
| Scan number | 200 | 200 | |
| Accumulation time (s) | 0.1 | 0.035 | |
| Transient length (s) | 3.35 | 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacroix-Andrivet, O.; Hubert-Roux, M.; Loutelier Bourhis, C.; Moualdi, S.; Mendes Siqueira, A.L.; Afonso, C. Characterization of Base Oil and Additive Oxidation Products from Formulated Lubricant by Ultra-High Resolution Mass Spectrometry. Lubricants 2023, 11, 345. https://doi.org/10.3390/lubricants11080345
Lacroix-Andrivet O, Hubert-Roux M, Loutelier Bourhis C, Moualdi S, Mendes Siqueira AL, Afonso C. Characterization of Base Oil and Additive Oxidation Products from Formulated Lubricant by Ultra-High Resolution Mass Spectrometry. Lubricants. 2023; 11(8):345. https://doi.org/10.3390/lubricants11080345
Chicago/Turabian StyleLacroix-Andrivet, Oscar, Marie Hubert-Roux, Corinne Loutelier Bourhis, Samira Moualdi, Anna Luiza Mendes Siqueira, and Carlos Afonso. 2023. "Characterization of Base Oil and Additive Oxidation Products from Formulated Lubricant by Ultra-High Resolution Mass Spectrometry" Lubricants 11, no. 8: 345. https://doi.org/10.3390/lubricants11080345