Elevated CO2 and Increased N Intensify Competition between Two Invasive Annual Plants in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Measurements and Calculations
2.4. Statistical Analysis
3. Results
3.1. Growth Characteristics of Two Invasive Alien Plants
3.2. Biomass Characteristics of Two Invasive Alien Plants
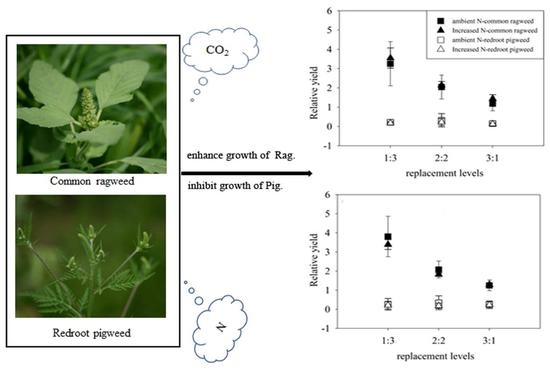
3.3. Relative Yields of Two Invasive Alien Species
3.4. Reproductive Characteristics of Common Ragweed
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; van Kleunen, M. Responses of common and rare aliens and natives to nutrient availability and fluctuations. J. Ecol. 2017, 105, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Oduor, A.M.O.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.; van Kleunen, M. Do invasive alien plants benefit more from global environmental change than native plants? Glob. Chang. Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef]
- Mozdzer, T.J.; Langley, J.A.; Mueller, P.; Megonigal, J.P. Deep rooting and global change facilitate spread of invasive grass. Biol. Invasions 2016, 18, 2619–2631. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Ibanez, I.; Blumenthal, D.M.; Molinari, N.A.; Miller, L.P.; Grosholz, E.D.; Diez, J.M.; D’Antonio, C.M.; Olden, J.D.; Jones, S.J.; et al. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 2013, 16, 261–270. [Google Scholar] [CrossRef]
- Dukes, J.S.; Chiariello, N.R.; Loarie, S.R.; Field, C.B. Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes. Ecol. Appl. 2011, 21, 1887–1894. [Google Scholar] [CrossRef] [Green Version]
- Ziska, L.H. Evaluation of the growth response of six invasive species to past, present and future atmospheric carbon dioxide. J. Exp. Bot. 2003, 54, 395–404. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Belote, R.T.; Sanders, N.J. Biological invaders in a greenhouse world: Will elevated CO2 fuel plant invasions? Front. Ecol. Environ. 2003, 1, 146–153. [Google Scholar] [CrossRef]
- Smith, S.D.; Huxman, T.E.; Zitzer, S.F.; Charlet, T.N.; Housman, D.C.; Coleman, J.S.; Fenstermaker, L.K.; Seemann, J.R.; Nowak, R.S. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 2000, 408, 79–82. [Google Scholar] [CrossRef]
- Stewart, J.; Potvin, C. Effects of elevated CO2 on an artificial grassland community: Competition, invasion and neighbourhood growth. Funct. Ecol. 1996, 10, 157–166. [Google Scholar] [CrossRef]
- Smith, S.D.; Charlet, T.N.; Zitzer, S.F.; Abella, S.R.; Vanier, C.H.; Huxman, T.E. Long-term response of a Mojave Desert winter annual plant community to a whole-ecosystem atmospheric CO2 manipulation (FACE). Glob. Change Biol. 2014, 20, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.-Y.; Qi, S.-S.; Zou, C.B.; Dai, Z.-C.; Ren, G.-Q.; Chen, Q.; Zhu, B.; Du, D.-L. Elevated nitrogen deposition may advance invasive weed, Solidago canadensis, in calcareous soils. J. Plant Ecol. 2019, 12, 846–856. [Google Scholar] [CrossRef]
- He, W.-M.; Yu, G.-L.; Sun, Z.-K. Nitrogen deposition enhances Bromus tectorum invasion: Biogeographic differences in growth and competitive ability between China and North America. Ecography 2011, 34, 1059–1066. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, W.; Cai, Z.; Valerie, P.; Han, F. Response of methane emission to invasion of Spartina alterniflora and exogenous N deposition in the coastal salt marsh. Atmos. Environ. 2010, 44, 4588–4594. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F.; Klees, H. N deposition affects N availability in interstitial water, growth of Sphagnum and invasion of vascular plants in bog vegetation. New Phytol. 2003, 157, 339–347. [Google Scholar] [CrossRef]
- Lei, Y.B.; Wang, W.B.; Feng, Y.L.; Zheng, Y.L.; Gong, H.D. Synergistic interactions of CO2 enrichment and nitrogen deposition promote growth and ecophysiological advantages of invading Eupatorium adenophorum in Southwest China. Planta 2012, 236, 1205–1213. [Google Scholar] [CrossRef]
- Nackley, L.; Hough-Snee, N.; Kim, S.H. Competitive traits of the invasive grass Arundo donax are enhanced by carbon dioxide and nitrogen enrichment. Weed Res. 2017, 57, 67–71. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Russell, J.C.; Sataruddin, N.S.; Heard, A.D. Over-invasion by functionally equivalent invasive species. Ecology 2014, 95, 2268–2276. [Google Scholar] [CrossRef]
- Kuebbing, S.E.; Nuñez, M.A. Negative, neutral, and positive interactions among nonnative plants: Patterns, processes, and management implications. Glob. Chang. Biol. 2015, 21, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Von Holle, B. Positive Interactions of Nonindigenous Species: Invasional Meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Jackson, M.C. Interactions among multiple invasive animals. Ecology 2015, 96, 2035–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauschert, E.S.J.; Shea, K. Competition between similar invasive species: Modeling invasional interference across a landscape. Popul. Ecol. 2017, 59, 79–88. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; Li, Z.; Zhu, S. Molecular Identification of a ‘Candidatus Phytoplasma ziziphi’-related Strain Infecting Amaranth (Amaranthus retroflexus L.) in China. J. Phytopathol. 2011, 159, 635–637. [Google Scholar] [CrossRef]
- Rauschert, E.S.J.; Shea, K. Invasional interference due to similar inter- and intraspecific competition between invaders may affect management. Ecol. Appl. 2012, 22, 1413–1420. [Google Scholar] [CrossRef]
- Wundrow, E.J.; Carrillo, J.; Gabler, C.A.; Horn, K.C.; Siemann, E. Facilitation and competition among invasive plants: A field experiment with alligatorweed and water hyacinth. PLoS ONE 2012, 7, e48444. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Y.; Li, H.; An, S.; Zhao, L.; Zhou, C.; Deng, Z. Inter-specific competition: Spartina alterniflora is replacing Spartina anglica in coastal China. Estuar. Coast. Shelf Sci. 2007, 74, 437–448. [Google Scholar] [CrossRef]
- Gong, L.; Li, J.S.; Liu, X.Y.; Zhao, X.J.; Zhao, C.Y. Analysis of invasive alien species in Chinese national nature reserves. Ecol. Sci. 2017, 36, 210–216. [Google Scholar] [CrossRef]
- Gentili, R.; Ambrosini, R.; Montagnani, C.; Caronni, S.; Citterio, S. Effect of Soil pH on the Growth, Reproductive Investment and Pollen Allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 2018, 9, 1335. [Google Scholar] [CrossRef]
- Ziska, L.H.; Caulfield, F.A. Rising CO2 and pollen production of common ragweed (Ambrosia artemisiifolia), a known allergy-inducing species: Implications for public health. Aust. J. Plant Physiol. 2000, 27, 893–898. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, J.S.; Zhao, C.Y.; Quan, Z.Z.; Zhao, X.J.; Gong, L. Prediction of potential suitable area of Ambrosia artemisiifolia L. in China based on MaxEnt and ArcGIS. J. Plant Proteciton 2016, 43, 1041–1048. [Google Scholar] [CrossRef]
- Valerio, M.; Tomecek, M.B.; Lovelli, S.; Ziska, L.H. Quantifying the effect of drought on carbon dioxide-induced changes in competition between a C3 crop (tomato) and a C4 weed (Amaranthus retroflexus). Weed Res. 2011, 51, 591–600. [Google Scholar] [CrossRef]
- Rezaie, F.; Yarnia, M. Allelopathic effects of Chenopodium album, Amaranthus retroflexus and Cynodon dactylon on germination and growth of safflower. J. Food Agric. Environ. 2009, 7, 516–521. [Google Scholar]
- Wang, C.; Zhou, J.; Liu, J.; Jiang, K. Differences in functional traits between invasive and native Amaranthus species under different forms of N deposition. Naturwissenschaften 2017, 104, 59. [Google Scholar] [CrossRef]
- Ma, J.; Xing, G.; Yang, W.; Ma, L.; Gao, M.; Wang, Y.; Han, Y. Inhibitory effects of leachate from Eupatorium adenophorum on germination and growth of Amaranthus retroflexus and Chenopodium glaucum. Acta Ecol. Sin. 2012, 32, 50–56. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; Jiang, Y.P.; Wei, H.; Wang, F.G.; Lu, X.N.; Clements, D. Invasion process and potential spread of Amaranthus retroflexus in China. Weed Res. 2018, 58, 57–67. [Google Scholar] [CrossRef]
- Marten, G.C.; Andersen, R.N. Forage Nutritive Value and Palatability of 12 Common Annual Weeds1. Crop Sci. 1975, 15, 821–827. [Google Scholar] [CrossRef]
- Lei, T.; Cui, G.F.; Sha, H.F.; Li, F.L.; Yan, J.H. Diversity and community distribution characteristics of alien vascular plant species in wetlands of Beijing. J. Beijign For. Univ. 2010, 32, 51–57. [Google Scholar] [CrossRef]
- IPCC. IPCC Fourth Assessment Report: Climate Change 2007. Available online: https://www.ipcc.ch/assessment-report/ar4/ (accessed on 9 October 2022).
- Galloway, J.; Townsend, A.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.; Martinelli, L.; Seitzinger, S.; Sutton, M. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Wang, J.; Sang, W. Effects of nitrogen deposition on invasive and competitive abilities of an alien plant Ambrosia artemisiifolia. J. Northeast Univ. 2012, 40, 60–66. [Google Scholar] [CrossRef]
- Williams, A.C.; McCarthy, B.C. A new index of interspecific competition for replacement and additive designs. Ecol. Res. 2001, 16, 29–40. [Google Scholar] [CrossRef]
- Weigelt, A.; Jolliffe, P. Indices of plant competition. J. Ecol. 2003, 91, 707–720. [Google Scholar] [CrossRef] [Green Version]
- Skalova, H.; Jarosik, V.; Dvorackova, S.; Pysek, P. Effect of Intra- and Interspecific Competition on the Performance of Native and Invasive Species of Impatiens under Varying Levels of Shade and Moisture. PLoS ONE 2013, 8, e62842. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wei, M.; Wang, S.; Wu, B.; Du, D. Plant height and leaf size: Which one is more important in affecting the successful invasion of Solidago canadensis and Conyza canadensis in urban ecosystems? Urban For. Urban Green. 2021, 59, 127033. [Google Scholar] [CrossRef]
- Rajcan, I.; AghaAlikhani, M.; Swanton, C.J.; Tollenaar, M. Development of redroot pigweed is influenced by light spectral quality and quantity. Crop Sci. 2002, 42, 1930–1936. [Google Scholar] [CrossRef]
- Chu, C.-C.; Ludford, P.M.; Ozbun, J.L.; Sweet, R.D. Effects of Temperature and Competition on the Establishment and Growth of Redroot Pigweed and Common Lambsquarters. Crop Sci. 1978, 18, 308–310. [Google Scholar] [CrossRef]
- Belote, R.T.; Weltzin, J.F. Interactions between two co-dominant, invasive plants in the understory of a temperate deciduous forest. Biol. Invasions 2006, 8, 1629–1641. [Google Scholar] [CrossRef]
- Griffen, B.D.; Guy, T.; Buck, J.C. Inhibition between invasives: A newly introduced predator moderates the impacts of a previously established invasive predator. J. Anim. Ecol. 2008, 77, 32–40. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Brandt, R.N.; Janzen, H.H.; Entz, T.; Grant, C.A.; Derksen, D.A. Differential response of weed species to added nitrogen. Weed Sci. 2003, 51, 532–539. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Kray, J.A.; Ortmans, W.; Ziska, L.H.; Pendall, E. Cheatgrass is favored by warming but not CO2 enrichment in a semi-arid grassland. Glob. Chang. Biol. 2016, 22, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- LeskovŠEk, R.; Datta, A.; Knezevic, S.Z.; SimonČIČ, A. Common ragweed (Ambrosia artemisiifolia) dry matter allocation and partitioning under different nitrogen and density levels. Weed Biol. Manag. 2012, 12, 98–108. [Google Scholar] [CrossRef]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of agricultural crops to free-air CO2 enrichment. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; Volume 77, pp. 293–368. [Google Scholar]
- Keser, L.H.; Dawson, W.; Song, Y.-B.; Yu, F.-H.; Fischer, M.; Dong, M.; van Kleunen, M. Invasive clonal plant species have a greater root-foraging plasticity than non-invasive ones. Oecologia 2014, 174, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keser, L.H.; Visser, E.J.; Dawson, W.; Song, Y.B.; Yu, F.H.; Fischer, M.; Dong, M.; van Kleunen, M. Herbaceous plant species invading natural areas tend to have stronger adaptive root foraging than other naturalized species. Front. Plant Sci. 2015, 6, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Ward, J.K.; Tissue, D.T.; Thomas, R.B.; Strain, B.R. Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Glob. Change Biol. 1999, 5, 857–867. [Google Scholar] [CrossRef]
- Weller, S.; Florentine, S.; Javaid, M.M.; Welgama, A.; Chadha, A.; Chauhan, B.S.; Turville, C. Amaranthus retroflexus L. (Redroot Pigweed): Effects of Elevated CO2 and Soil Moisture on Growth and Biomass and the Effect of Radiant Heat on Seed Germination. Agron. Basel 2021, 11, 728. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Ehleringer, J. Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ. 1984, 7, 1–13. [Google Scholar] [CrossRef]
- Poorter, H. Interspecific Variation in the growth-response of plants to an elevated ambient CO2 concentration. Vegetatio 1993, 104, 77–97. [Google Scholar] [CrossRef]
- Bae, J.; Byun, C.; Gyong, A.Y.; Choi, J.H.; Lee, D.; Kang, H. Effect of elevated atmospheric carbon dioxide on the allelopathic potential of common ragweed. J. Ecol. Environ. 2019, 43, 212–218. [Google Scholar] [CrossRef]
- Lonsdale, W.M. Global patterns of plant invasions and the concept of invasibility. Ecology 1999, 80, 1522–1536. [Google Scholar] [CrossRef]
- Leskovšek, R.; Eler, K.; Batič, F.; Simončič, A. The influence of nitrogen, water and competition on the vegetative and reproductive growth of common ragweed (Ambrosia artemisiifolia L.). Plant Ecol. 2012, 213, 769–781. [Google Scholar] [CrossRef]
- Amini, R.; Alizadeh, H.; Yousefi, A. Interference between red kidneybean (Phaseolus vulgaris L.) cultivars and redroot pigweed (Amaranthus retroflexus L.). Eur. J. Agron. 2014, 60, 13–21. [Google Scholar] [CrossRef]
- Golivets, M.; Wallin, K.F. Neighbour tolerance, not suppression, provides competitive advantage to non-native plants. Ecol. Lett. 2018, 21, 745–759. [Google Scholar] [CrossRef] [Green Version]
- HilleRisLambers, J.; Harpole, W.S.; Tilman, D.; Knops, J.; Reich, P.B. Mechanisms responsible for the positive diversity-productivity relationship in Minnesota grasslands. Ecol. Lett. 2004, 7, 661–668. [Google Scholar] [CrossRef]




| Species | Factors | Basal Stem Diameter | Height | Branch Number | |
|---|---|---|---|---|---|
| common ragweed | CO2 | F | 2.756 | 3.881 | 0.84 |
| p | 0.104 | 0.055 | 0.364 | ||
| df | 1 | 1 | 1 | ||
| N | F | 32.843 | 17.834 | 0.077 | |
| p | <0.001 ** | <0.001 ** | 0.783 | ||
| df | 1 | 1 | 1 | ||
| replacement | F | 14.961 | 2.446 | 15.668 | |
| p | <0.001 ** | 0.076 | <0.001 ** | ||
| df | 3 | 3 | 3 | ||
| CO2 × N | F | 0.102 | 3.65 | 2.025 | |
| p | 0.751 | 0.062 | 0.161 | ||
| df | 1 | 1 | 1 | ||
| N × replacement | F | 1.163 | 0.398 | 2.198 | |
| p | 0.334 | 0.755 | 0.101 | ||
| df | 3 | 3 | 3 | ||
| CO2 × replacement | F | 1.093 | 0.222 | 0.905 | |
| p | 0.361 | 0.881 | 0.446 | ||
| df | 3 | 3 | 3 | ||
| CO2 × N × replacement | F | 0.128 | 0.944 | 1.094 | |
| p | 0.943 | 0.427 | 0.361 | ||
| df | 3 | 3 | 3 | ||
| redroot pigweed | CO2 | F | 0 | 3.405 | - |
| p | 0.994 | 0.071 | - | ||
| df | 1 | 1 | |||
| N | F | 4.599 | 26.336 | - | |
| p | 0.037 * | <0.001 ** | - | ||
| df | 1 | 1 | |||
| replacement | F | 36.172 | 43.233 | - | |
| p | <0.001 ** | <0.001 ** | - | ||
| df | 3 | 3 | |||
| CO2 × N | F | 0.009 | 0.249 | - | |
| p | 0.926 | 0.62 | - | ||
| df | 1 | 1 | |||
| N × replacement | F | 2.971 | 0.834 | - | |
| p | 0.041 * | 0.482 | - | ||
| df | 3 | 3 | |||
| CO2 × replacement | F | 0.896 | 3.64 | - | |
| p | 0.45 | 0.019 * | - | ||
| df | 3 | 3 | |||
| CO2 × N × replacement | F | 0.289 | 0.712 | - | |
| p | 0.833 | 0.55 | - | ||
| df | 3 | 3 |
| Species | Factors | Shoot Biomass | Root Biomass | Total Biomass | Root-Shoot Ratio | |
|---|---|---|---|---|---|---|
| common ragweed | CO2 | F | 2.141 | 0.855 | 2.107 | 0.042 |
| p | 0.15 | 0.36 | 0.153 | 0.839 | ||
| df | 1 | 1 | 1 | 1 | ||
| N | F | 140.242 | 73.943 | 142.018 | 2.843 | |
| p | <0.001 ** | <0.001 ** | <0.001 ** | 0.099 | ||
| df | 1 | 1 | 1 | 1 | ||
| replacement | F | 1.085 | 5.75 | 1.398 | 5.158 | |
| p | 0.365 | 0.002 ** | 0.255 | 0.004 ** | ||
| df | 3 | 3 | 3 | 3 | ||
| CO2 × N | F | 13.565 | 4.989 | 13.245 | 2.609 | |
| p | 0.001 ** | 0.030 * | 0.001 ** | 0.113 | ||
| df | 1 | 1 | 1 | 1 | ||
| N × replacement | F | 0.399 | 2.804 | 0.651 | 1.275 | |
| p | 0.754 | 0.05 | 0.587 | 0.294 | ||
| df | 3 | 3 | 3 | 3 | ||
| CO2 × replacement | F | 0.361 | 0.808 | 0.346 | 0.806 | |
| p | 0.781 | 0.496 | 0.792 | 0.497 | ||
| df | 3 | 3 | 3 | 3 | ||
| CO2 × N × replacement | F | 0.354 | 0.064 | 0.271 | 0.294 | |
| p | 0.786 | 0.979 | 0.846 | 0.83 | ||
| df | 3 | 3 | 3 | 3 | ||
| redroot pigweed | CO2 | F | 1.92 | 5.08 | 2.63 | 0.037 |
| p | 0.173 | 0.029 * | 0.112 | 0.849 | ||
| df | 1 | 1 | 1 | 1 | ||
| N | F | 1.614 | 0.013 | 1.637 | 0.383 | |
| p | 0.21 | 0.909 | 0.207 | 0.539 | ||
| df | 1 | 1 | 1 | 1 | ||
| replacement | F | 100.968 | 57.074 | 110.475 | 4.281 | |
| p | <0.001 ** | <0.001 ** | <0.001 ** | 0.010 ** | ||
| df | 3 | 3 | 3 | 3 | ||
| CO2 × N | F | 0.588 | 0.054 | 0.636 | 0.088 | |
| p | 0.447 | 0.818 | 0.429 | 0.768 | ||
| df | 1 | 1 | 1 | 1 | ||
| N × replacement | F | 0.512 | 0.182 | 0.472 | 0.184 | |
| p | 0.676 | 0.908 | 0.703 | 0.907 | ||
| df | 3 | 3 | 3 | 3 | ||
| CO2 × replacement | F | 0.491 | 0.597 | 0.464 | 1.36 | |
| p | 0.69 | 0.62 | 0.709 | 0.267 | ||
| df | 3 | 3 | 3 | 3 | ||
| CO2 × N × replacement | F | 0.033 | 0.374 | 0.027 | 0.736 | |
| p | 0.992 | 0.772 | 0.994 | 0.536 | ||
| df | 3 | 3 | 3 | 3 |
| Factors | Seed Number | Seed Total Weight | Seed Mean Weight | |
|---|---|---|---|---|
| CO2 | F | 0.017 | 0.344 | 0.181 |
| p | 0.896 | 0.560 | 0.673 | |
| df | 1 | 1 | 1 | |
| N | F | 14.821 | 1.241 | 12.024 |
| p | <0.001 ** | 0.271 | 0.001 ** | |
| df | 1 | 1 | 1 | |
| replacement | F | 2.316 | 2.135 | 0.330 |
| p | 0.088 | 0.109 | 0.804 | |
| df | 3 | 3 | 3 | |
| CO2 × N | F | 1.654 | 0.999 | 0.092 |
| p | 0.205 | 0.323 | 0.763 | |
| df | 1 | 1 | 1 | |
| N × replacement | F | 0.437 | 0.926 | 0.689 |
| p | 0.727 | 0.436 | 0.563 | |
| df | 3 | 3 | 3 | |
| CO2 × replacement | F | 0.806 | 0.360 | 0.083 |
| p | 0.497 | 0.782 | 0.969 | |
| df | 3 | 3 | 3 | |
| CO2 × N × replacement | F | 1.203 | 0.977 | 1.885 |
| p | 0.319 | 0.412 | 0.145 | |
| df | 3 | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhao, X.; Li, J. Elevated CO2 and Increased N Intensify Competition between Two Invasive Annual Plants in China. Life 2022, 12, 1669. https://doi.org/10.3390/life12101669
Zhao C, Zhao X, Li J. Elevated CO2 and Increased N Intensify Competition between Two Invasive Annual Plants in China. Life. 2022; 12(10):1669. https://doi.org/10.3390/life12101669
Chicago/Turabian StyleZhao, Caiyun, Xiangjian Zhao, and Junsheng Li. 2022. "Elevated CO2 and Increased N Intensify Competition between Two Invasive Annual Plants in China" Life 12, no. 10: 1669. https://doi.org/10.3390/life12101669