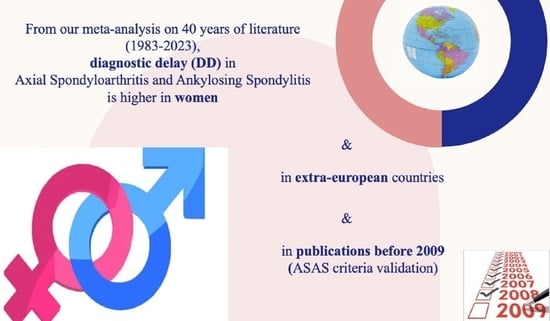
Sex Bias in Diagnostic Delay: Are Axial Spondyloarthritis and Ankylosing Spondylitis Still Phantom Diseases in Women? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process
2.4. Statistical Analysis
3. Results
3.1. HLA*B27
3.2. Clinical Presentation
3.3. Social Parameters
3.4. Meta-Analysis of DD Difference between Sexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cutolo, M.; Straub, R.H. Sex Steroids and Autoimmune Rheumatic Diseases: State of the Art. Nat. Rev. Rheumatol. 2020, 16, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, M.-S.; Alten, R.; D’Agostino, M.-A.; Gremese, E.; Kiltz, U.; Lubrano, E.; Moreno, M.; Pham, T.; Ramonda, R.; Spinelli, F.-R. Sex-Associated and Gender-Associated Differences in the Diagnosis and Management of Axial Spondyloarthritis: Addressing the Unmet Needs of Female Patients. RMD Open 2021, 7, e001681. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.A.; Crowson, C.S.; Michet, C.J.; Matteson, E.L. Time Trends in Incidence, Clinical Features, and Cardiovascular Disease in Ankylosing Spondylitis over Three Decades: A Population-Based Study. Arthritis Care Res. 2015, 67, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Siragusano, C.; Alciati, A.; Cassone, G.; D’Angelo, S.; Guiducci, S.; Favalli, E.G.; Conti, F.; Gremese, E.; Iannone, F.; et al. Axial Spondyloarthritis: Reshape the Future-From the “2022 GISEA International Symposium”. J. Clin. Med. 2022, 11, 7537. [Google Scholar] [CrossRef] [PubMed]
- Benagiano, M.; Bianchi, P.; D’Elios, M.M.; Brosens, I.; Benagiano, G. Autoimmune Diseases: Role of Steroid Hormones. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.C. Nonendocrine Mechanisms of Sex Bias in Rheumatic Diseases. Nat. Rev. Rheumatol. 2019, 15, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Feldtkeller, E.; Braun, J. Impact of Sex on Inheritance of Ankylosing Spondylitis. Lancet 2000, 355, 1096–1097. [Google Scholar] [CrossRef]
- Pradeep, D.J.; Keat, A.; Gaffney, K. Predicting Outcome in Ankylosing Spondylitis. Rheumatology 2008, 47, 942–945. [Google Scholar] [CrossRef]
- Diaconu, A.-D.; Ceasovschih, A.; Șorodoc, V.; Pomîrleanu, C.; Lionte, C.; Șorodoc, L.; Ancuța, C. Practical Significance of Biomarkers in Axial Spondyloarthritis: Updates on Diagnosis, Disease Activity, and Prognosis. Int. J. Mol. Sci. 2022, 23, 11561. [Google Scholar] [CrossRef]
- Adshead, R.; Donnelly, S.; Knight, P.; Tahir, H. Axial Spondyloarthritis: Overcoming the Barriers to Early Diagnosis-an Early Inflammatory Back Pain Service. Curr. Rheumatol. Rep. 2020, 22, 59. [Google Scholar] [CrossRef]
- Salvadorini, G.; Bandinelli, F.; Delle Sedie, A.; Riente, L.; Candelieri, A.; Generini, S.; Possemato, N.; Bombardieri, S.; Matucci-Cerinic, M. Ankylosing Spondylitis: How Diagnostic and Therapeutic Delay Have Changed over the Last Six Decades. Clin. Exp. Rheumatol. Incl. Suppl. 2012, 30, 561. [Google Scholar]
- Neto, A.; Figueira, R.; Quintal, A.; Rodrigues, M. The Natural Progression of Ankylosing Spondylitis After 4 Decades of Untreated Disease. J. Clin. Rheumatol. 2021, 27, S783. [Google Scholar] [CrossRef] [PubMed]
- Feldtkeller, E.; Erlendsson, J. Definition of Disease Duration in Ankylosing Spondylitis. Rheumatol. Int. 2008, 28, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Pittam, B.; Harrison, N.L.; Ahmed, A.E.; Goodson, N.J.; Hughes, D.M. Diagnostic Delay in Axial Spondyloarthritis: A Systematic Review and Meta-Analysis. Rheumatology 2021, 60, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; van der Heijde, D.; Landewe, R.; Brandt, J.; Burgos-Vagas, R.; Collantes-Estevez, E.; Dijkmans, B.; Dougados, M.; Khan, M.; Leirisalo-Repo, M. New Criteria for Inflammatory Back Pain in Patients with Chronic Back Pain: A Real Patient Exercise by Experts from the Assessment of SpondyloArthritis International Society (ASAS). Ann. Rheum. Dis. 2009, 68, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, F.; Delle Sedie, A.; Salvadorini, G.; Riente, L.; Candelieri, A.; Generini, S.; Bombardieri, S. Reply to Comment on ‘Ankylosing Spondylitis: How Diagnostic and Therapeutic Delay Have Changed over the Last Six Decades’ E. Feldtkeller, A. Zeller, M. Rudwaleit. Clin. Exp. Rheumatol. 2013, 31, 993. [Google Scholar] [PubMed]
- Bandinelli, F.; Terenzi, R.; Giovannini, L.; Milla, M.; Genise, S.; Bagnoli, S.; Biagini, S.; Annese, V.; Matucci-Cerinic, M. Occult Radiological Sacroiliac Abnormalities in Patients with Inflammatory Bowel Disease Who Do Not Present Signs or Symptoms of Axial Spondylitis. Clin. Exp. Rheumatol. 2014, 32, 949–952. [Google Scholar]
- Bandinelli, F.; Melchiorre, D.; Scazzariello, F.; Candelieri, A.; Conforti, D.; Matucci-Cerinic, M. Clinical and Radiological Evaluation of Sacroiliac Joints Compared with Ultrasound Examination in Early Spondyloarthritis. Rheumatology 2013, 52, 1293–1297. [Google Scholar] [CrossRef]
- Tas, N.P.; Kaya, O.; Macin, G.; Tasci, B.; Dogan, S.; Tuncer, T. ASNET: A Novel AI Framework for Accurate Ankylosing Spondylitis Diagnosis from MRI. Biomedicines 2023, 11, 2441. [Google Scholar] [CrossRef]
- Benucci, M.; Damiani, A.; Bandinelli, F.; Russo, E.; Li Gobbi, F.; Grossi, V.; Amedei, A.; Infantino, M.; Manfredi, M. Correction: Benucci et al. Predicting Loss of Efficacy after Non-Medical Switching: Correlation between Circulating TNF-α Levels and SB4 in Etanercept to SB4 Switchers and Naïve Patients with Rheumatic Disease. J. Pers. Med. 2022, 12, 1174, J. Pers. Med.2023, 13, 168. . [Google Scholar] [CrossRef]
- Favalli, E.G.; Becciolini, A.; Caporali, R.; Todoerti, M.; Iannone, F.; Dinoia, L.; Sebastiani, M.; Spinella, A.; Gremese, E.; Cianci, F.; et al. The Profiling of Axial Spondyloarthritis Patient Candidate to a Biologic Therapy: Consensus from a Delphi-Panel of Italian Experts. Autoimmun. Rev. 2018, 17, 1251–1258. [Google Scholar] [CrossRef]
- Jovani, V.; Blasco-Blasco, M.; Pascual, E.; Ruiz-Cantero, M.T. Challenges to Conquer from the Gender Perspective in Medicine: The Case of Spondyloarthritis. PLoS ONE 2018, 13, e0205751. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; López-Medina, C.; Khan, M.A.; Dougados, M.; Magrey, M. Clinical Profile and Treatment Utilisation Based on HLA-B*27 Status in Axial Spondyloarthritis: Results from ASAS-PerSpA Study. RMD Open 2023, 9, e003179. [Google Scholar] [CrossRef] [PubMed]
- Hajialilo, M.; Ghorbanihaghjo, A.; Khabbazi, A.; Kolahi, S.; Rashtchizadeh, N. Ankylosing Spondylitis in Iran; Late Diagnosis and Its Causes. Iran. Red. Crescent Med. J. 2014, 16, e11798. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chen, J.; Tu, J.; Ye, W.; Zhang, Z.; Liu, Q.; Zhu, X. Association of HLA-B27 Status and Gender with Sacroiliitis in Patients with Ankylosing Spondylitis. Pak. J. Med. Sci. 2014, 30, 22. [Google Scholar] [PubMed]
- Min, H.K.; Cho, H.; Park, S.-H. Baseline Severity of Sacroiliitis Can Predict Acute Inflammatory Status of Sacroiliac Joint in Early Axial Spondyloarthritis of Male Patients: A Cross Sectional Study. BMC Musculoskelet. Disord. 2019, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Ensslin, C.; Micheroli, R.; Kissling, S.; Götschi, A.; Bürki, K.; Bräm, R.; de Hooge, M.; Baraliakos, X.; Nissen, M.J.; Möller, B. Impact of Sex on Spinal Radiographic Progression in Axial Spondyloarthritis: A Longitudinal Swiss Cohort Analysis over a Period of 10 Years. RMD Open 2023, 9, e003340. [Google Scholar] [CrossRef]
- Bandinelli, F.; Manetti, M.; Ibba-Manneschi, L. Occult Spondyloarthritis in Inflammatory Bowel Disease. Clin. Rheumatol. 2016, 35, 281–289. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of Diagnostic Criteria for Ankylosing Spondylitis. A Proposal for Modification of the New York Criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Amor, B.; Dougados, M.; Mijiyawa, M. Criteria of the classification of spondylarthropathies. Rev. Rhum. Mal. Osteoartic. 1990, 57, 85–89. [Google Scholar] [PubMed]
- Cherkin, D.C.; Deyo, R.A.; Volinn, E.; Loeser, J.D. Use of the International Classification of Diseases (ICD-9-CM) to Identify Hospitalizations for Mechanical Low Back Problems in Administrative Databases. Spine 1992, 17, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; van der Linden, S.; Juhlin, R.; Huitfeldt, B.; Amor, B.; Calin, A.; Cats, A.; Dijkmans, B.; Olivieri, I.; Pasero, G. The European Spondylarthropathy Study Group Preliminary Criteria for the Classification of Spondylarthropathy. Arthritis Rheum. 1991, 34, 1218–1227. [Google Scholar] [CrossRef]
- Vassar, M.; Atakpo, P.; Kash, M.J. Manual Search Approaches Used by Systematic Reviewers in Dermatology. J. Med. Libr. Assoc. 2016, 104, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Jovaní, V.; Blasco-Blasco, M.; Ruiz-Cantero, M.T.; Pascual, E. Understanding How the Diagnostic Delay of Spondyloarthritis Differs between Women and Men: A Systematic Review and Metaanalysis. J. Rheumatol. 2017, 44, 174–183. [Google Scholar] [CrossRef]
- Calin, A.; Porta, J.; Fries, J.F.; Schurman, D.J. Clinical History as a Screening Test for Ankylosing Spondylitis. Jama 1977, 237, 2613–2614. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Malaviya, A.N. Diagnosis Delay in Patients with Ankylosing Spondylitis: Factors and Outcomes—An Indian Perspective. Clin. Rheumatol. 2009, 28, 327–331. [Google Scholar] [CrossRef]
- Almousa, S.; Alshamaa, N.; Wannous, H.; Khder, K.; Qasem, H. Gender-Related Differences in Axial Spondyloarthritis (axSpA) Patients. Egypt. Rheumatol. 2023, 45, 13–16. [Google Scholar] [CrossRef]
- Aloush, V.; Ablin, J.N.; Reitblat, T.; Caspi, D.; Elkayam, O. Fibromyalgia in Women with Ankylosing Spondylitis. Rheumatol. Int. 2007, 27, 865–868. [Google Scholar] [CrossRef]
- Atagunduz, P.; Aydin, S.Z.; Bahadir, C.; Erer, B.; Direskeneli, H. Determinants of Early Radiographic Progression in Ankylosing Spondylitis. J. Rheumatol. 2010, 37, 2356–2361. [Google Scholar] [CrossRef]
- Bakland, G.; Gran, J.T.; Nossent, J.C. Increased Mortality in Ankylosing Spondylitis Is Related to Disease Activity. Ann. Rheum. Dis. 2011, 70, 1921–1925. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, F.; Salvadorini, G.; Sedie, A.D.; Riente, L.; Bombardieri, S.; Matucci-Cerinic, M. Impact of Gender, Work, and Clinical Presentation on Diagnostic Delay in Italian Patients with Primary Ankylosing Spondylitis. Clin. Rheumatol. 2016, 35, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Blasco, M.; Ruiz-Cantero, M.T.; Juárez-Herrera y Cairo, L.A.; Jovaní, V.; Pascual, E. Sex and Gender Interactions in the Lives of Patients with Spondyloarthritis in Spain: A Quantitative-Qualitative Study. J. Rheumatol. 2017, 44, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Bodur, H.; Ataman, Ş.; Buğdaycı, D.S.; Rezvani, A.; Nas, K.; Uzunca, K.; Emlakçıoğlu, E.; Karatepe, A.G.; Durmuş, B.; Sezgin, M. Description of the Registry of Patients with Ankylosing Spondylitis in Turkey: TRASD-IP. Rheumatol. Int. 2012, 32, 169–176. [Google Scholar] [CrossRef]
- Deodhar, A.; Mittal, M.; Reilly, P.; Bao, Y.; Manthena, S.; Anderson, J.; Joshi, A. Ankylosing Spondylitis Diagnosis in US Patients with Back Pain: Identifying Providers Involved and Factors Associated with Rheumatology Referral Delay. Clin. Rheumatol. 2016, 35, 1769–1776. [Google Scholar] [CrossRef]
- Dincer, U.; Cakar, E.; Kiralp, M.Z.; Dursun, H. Diagnosis Delay in Patients with Ankylosing Spondylitis: Possible Reasons and Proposals for New Diagnostic Criteria. Clin. Rheumatol. 2008, 27, 457–462. [Google Scholar] [CrossRef]
- Garrido-Cumbrera, M.; Navarro-Compan, V.; Bundy, C.; Mahapatra, R.; Makri, S.; Correa-Fernandez, J.; Christen, L.; Delgado-Domínguez, C.J.; Poddubnyy, D.; EMAS Working Group. Identifying Parameters Associated with Delayed Diagnosis in Axial Spondyloarthritis: Data from the European Map of Axial Spondyloarthritis. Rheumatology 2022, 61, 705–712. [Google Scholar] [CrossRef]
- Ibn Yacoub, Y.; Amine, B.; Laatiris, A.; Bensabbah, R.; Hajjaj-Hassouni, N. Relationship between Diagnosis Delay and Disease Features in Moroccan Patients with Ankylosing Spondylitis. Rheumatol. Int. 2012, 32, 357–360. [Google Scholar] [CrossRef]
- Landi, M.; Maldonado-Ficco, H.; Perez-Alamino, R.; Maldonado-Cocco, J.A.; Citera, G.; Arturi, P.; Sampaio-Barros, P.D.; Alvarado, D.E.F.; Burgos-Vargas, R.; Santos, E. Gender Differences among Patients with Primary Ankylosing Spondylitis and Spondylitis Associated with Psoriasis and Inflammatory Bowel Disease in an Iberoamerican Spondyloarthritis Cohort. Medicine 2016, 95, e5652. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Chen, Y.; Ye, C.; Huang, J.; Qian, L.; Xin, W.; Li, T.; Ye, S. A Multidisciplinary Clinic Approach to Improve Physician-Related Diagnostic Delay for Patients with Axial Spondyloarthritis: A Retrospective Study. J. Int. Med. Res. 2019, 47, 2483–2491. [Google Scholar] [CrossRef]
- Ma, H.; Yin, Q.; Hu, F.; Guo, M.; Liu, X.; Liu, Y.; Xu, Q. Different Clinical Features in Patients with Ankylosing Spondylitis from Southern and Northern China. Int. J. Rheum. Dis. 2012, 15, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.; Barnett, M.; Calin, A. Ankylosing Spondylitis in Women and Men: A Case-Control Study. J. Rheumatol. 1983, 10, 624–628. [Google Scholar] [PubMed]
- Neuenschwander, R.; Hebeisen, M.; Micheroli, R.; Bürki, K.; Exer, P.; Niedermann, K.; Nissen, M.J.; Scherer, A.; Ciurea, A. Differences between Men and Women with Nonradiographic Axial Spondyloarthritis: Clinical Characteristics and Treatment Effectiveness in a Real-Life Prospective Cohort. Arthritis Res. Ther. 2020, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Benjamin Nowell, W.; Reynolds, R.; Gavigan, K.; Venkatachalam, S.; de la Cruz, M.; Flood, E.; Schwartz, E.J.; Romero, B.; Park, Y. Real-World Patient Experience on the Path to Diagnosis of Ankylosing Spondylitis. Rheumatol. Ther. 2019, 6, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Dharmage, S.; Boers, A.; Martin, B.J.; Buchanan, R.R.; Schachna, L. Ankylosing Spondylitis: An Australian Experience. Intern. Med. J. 2008, 38, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ringsdal, V.S.; Andreasen, J.J. Ankylosing Spondylitis—Experience with a Self Administered Questionnaire: An Analytical Study. Ann. Rheum. Dis. 1989, 48, 924–927. [Google Scholar] [CrossRef]
- Roussou, E.; Sultana, S. Spondyloarthritis in Women: Differences in Disease Onset, Clinical Presentation, and Bath Ankylosing Spondylitis Disease Activity and Functional Indices (BASDAI and BASFI) between Men and Women with Spondyloarthritides. Clin. Rheumatol. 2011, 30, 121–127. [Google Scholar] [CrossRef]
- Shahlaee, A.; Mahmoudi, M.; Nicknam, M.H.; Farhadi, E.; Fallahi, S.; Jamshidi, A.R. Gender Differences in Iranian Patients with Ankylosing Spondylitis. Clin. Rheumatol. 2015, 34, 285–293. [Google Scholar] [CrossRef]
- Slobodin, G.; Reyhan, I.; Avshovich, N.; Balbir-Gurman, A.; Boulman, N.; Elias, M.; Feld, J.; Mader, R.; Markovitz, D.; Rimar, D.; et al. Recently Diagnosed Axial Spondyloarthritis: Gender Differences and Factors Related to Delay in Diagnosis. Clin. Rheumatol. 2011, 30, 1075–1080. [Google Scholar] [CrossRef]
- Zink, A.; Braun, J.; Listing, J.; Wollenhaupt, J. Disability and Handicap in Rheumatoid Arthritis and Ankylosing Spondylitis—Results from the German Rheumatological Database. German Collaborative Arthritis Centers. J. Rheumatol. 2000, 27, 613–622. [Google Scholar]
- Higgins, J.; Li, T.; Deeks, J. Chapter 6. Section 6.5.2.8: Imputing Standard Deviations for Changes from Baseline. In Cochrane Handbook for Systematic Reviews of Interventions Version 6; The Cochrane Collaboration: London, UK, 2019. [Google Scholar]
- von Hippel, P.T. The Heterogeneity Statistic I(2) Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Li, T.; Deeks, J. Chapter 10. Section 10.10.2: Identifying and measuring heterogeneity. In Cochrane Handbook for Systematic Reviews of Interventions Version 6; The Cochrane Collaboration: London, UK, 2019. [Google Scholar]
- Fioravanti, M.; Yanagi, M. Cytidinediphosphocholine (CDP Choline) for Cognitive and Behavioural Disturbances Associated with Chronic Cerebral Disorders in the Elderly. Cochrane Database Syst. Rev. 2000, 2, CD000269. [Google Scholar] [CrossRef]
- Khan, M.A. An Update on the Genetic Polymorphism of HLA-B*27 with 213 Alleles Encompassing 160 Subtypes (and Still Counting). Curr. Rheumatol. Rep. 2017, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, J.; Li, X.; Wei, Q.; Lv, Q.; Zhang, P.; Zheng, X.; Chen, Z.; Cao, S.; Tu, L.; et al. The Clinical Characteristics of Other HLA-B Types in Chinese Ankylosing Spondylitis Patients. Front. Med. 2020, 7, 568790. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.M.; Alunno, A.; Di Ruscio, E.; Altieri, P.; Ferri, C.; Carubbi, F. Human Leukocyte Antigen B*27-Negative Spondyloarthritis: Clinical, Serological, and Radiological Features of a Single-Center Cohort. Diagnostics 2023, 13, 3550. [Google Scholar] [CrossRef]
- Paladini, F.; Fiorillo, M.T.; Tedeschi, V.; Cauli, A.; Mathieu, A.; Sorrentino, R. Ankylosing Spondylitis: A Trade Off of HLA-B27, ERAP, and Pathogen Interconnections? Focus on Sardinia. Front. Immunol. 2019, 10, 35. [Google Scholar] [CrossRef]
- Wu, X.; Wang, G.; Zhang, L.; Xu, H. Genetics of Ankylosing Spondylitis-Focusing on the Ethnic Difference Between East Asia and Europe. Front. Genet. 2021, 12, 671682. [Google Scholar] [CrossRef]
- Díaz-Peña, R.; Castro-Santos, P.; Durán, J.; Santiago, C.; Lucia, A. The Genetics of Spondyloarthritis. J. Pers. Med. 2020, 10, 151. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Gao, R.; Zhou, Y.; Lai, C.; Li, Z.; Xian, W.; Qian, X.; Li, Z.; Huang, Y. Initial Whole-Genome Sequencing and Analysis of the Host Genetic Contribution to COVID-19 Severity and Susceptibility. Cell Discov. 2020, 6, 83. [Google Scholar] [CrossRef]
- Wang, C.-M.; Tan, K.-P.; Jan Wu, Y.-J.; Lin, J.-C.; Zheng, J.-W.; Yu, A.L.; Wu, J.-M.; Chen, J.-Y. MICA*019 Allele and Soluble MICA as Biomarkers for Ankylosing Spondylitis in Taiwanese. J. Pers. Med. 2021, 11, 564. [Google Scholar] [CrossRef]
- Deodhar, A.; Gill, T.; Magrey, M. Human Leukocyte Antigen B27-Negative Axial Spondyloarthritis: What Do We Know? ACR Open Rheumatol. 2023, 5, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.; Couto, A.R.; Foroni, I.; Bettencourt, B.F.; Li, Z.; Meneses, R.; Wheeler, L.; Pereira, J.; Pimentel-Santos, F.; Fonseca, J.E.; et al. Non-Classical Human Leucocyte Antigens in Ankylosing Spondylitis: Possible Association with HLA-E and HLA-F. RMD Open 2018, 4, e000677. [Google Scholar] [CrossRef]
- Yen, Y.-N.; Garrido-Cumbrera, M.; Sun, Y.-S.; Chen, C.-H.; Lai, C.-C.; Tsai, H.-C.; Chen, W.-S.; Liao, H.-T.; Tsao, Y.-P.; Ankylosing Spondylitis Caring Society of ROC (ASCARES); et al. The Taiwanese Map of Axial Spondyloarthritis: Living with the Condition. Medicina 2023, 59, 1962. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Li, M.-M. Ankylosing Spondylitis and Psychiatric Disorders in European Population: A Mendelian Randomization Study. Front. Immunol. 2023, 14, 1277959. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.R.M.; Goyal, B.; Mamgain, G.; Kothari, A.; Kumar, S.; Saha, S.; Naithani, M.; Mirza, A.A.; Kumar, R.; Arora, R. Genetic Variations in IL-1β, TNF-α, and TGF-β Associated with the Severity of Chronic Cervical Spondylitis in Patients. Cells 2023, 12, 1594. [Google Scholar] [CrossRef]
- Canossi, A.; Oumhani, K.; Del Beato, T.; Sebastiani, P.; Colanardi, A.; Aureli, A. The Role of CD1 Gene Polymorphism in the Genetic Susceptibility to Spondyloarthropathies in the Moroccan Population and the Possible Cross-Link with Celiac Disease. Vaccines 2023, 11, 237. [Google Scholar] [CrossRef]
- Mathieu, A.; Cauli, A.; Fiorillo, M.T.; Sorrentino, R. HLA-B27 and Ankylosing Spondylitis Geographic Distribution as the Result of a Genetic Selection Induced by Malaria Endemic? A Review Supporting the Hypothesis. Autoimmun. Rev. 2008, 7, 398–403. [Google Scholar] [CrossRef]
- Bugaj, B.; Wielińska, J.; Bogunia-Kubik, K.; Świerkot, J. Searching for New Genetic Biomarkers of Axial Spondyloarthritis. J. Clin. Med. 2022, 11, 2912. [Google Scholar] [CrossRef]





| Study | Disease | Classification Criteria | Sample Size and F:M Ratio | Sample Source | WB Class | Mean Age at Symptom Onset F vs. M | Mean Age at Diagnosis F vs. M | Mean Delay to Diagnosis F vs. M |
|---|---|---|---|---|---|---|---|---|
| Aggarwal 2009 [37] IND | AS | mNY | 70 11:59 | SC | Lower-middle | NR | NR | 8.6 (6.6) vs. 6.5 (4.7) p = 0.2 |
| Almousa 2023 [38] SYR | ax-Spa | ASAS | 114 38:76 | MC | Lower-middle | 25.0 (5.1) vs. 25.1 (6.1) p = 0.9 | NR | 9.2 (3.9) vs. 6.7 (3.4) p = 0.001 |
| Aloush 2007 [39] ISR | AS | mNY | 36 18:18 | SC | High | NR | NR | 9.9 vs. 4.1 p = 0.05 |
| Atagunduz 2010 [40] TUR | AS | mNY | 235 96:139 | MC | Upper-middle | 29.7 (10.2) vs. 25.1 (9.0) p = 0.87 | NR | 7.4 (7.9) vs. 6.2 (7.1) p = 0.8 |
| Bakland 2011 [41] EUR | AS | mNY | 677 166:511 | SC | High | NA | NR | p = NS |
| Bandinelli 2016 [42] EUR | ax-SpA | ASAS/ mNY | 135 44:91 | SC | High | NA | NA | 6.3 (1.1) vs. 9.9 (0.8) p = 0.002 |
| Blasco-Blasco 2017 [43] EUR | SpA | Self-reported ASAS | 145 52:93 | SC | High | NR | NR | p = 0.1 (in patients with DD impact on work) |
| Bodur 2012 [44] TUR | AS | mNY | 1381 343:1038 | NT | Upper-middle | 30.4 (10.0) vs. 26.5 (9.5) p = 0.000 | NA | 5.3 (7.0) vs. 4.9 (6.9) p = 0.4 |
| Deodhar 2016 [45] USA | AS | ICD-9-CM | 3336 1677: 1659 | NT | High | NR | NR | NR p = 0.016 |
| Dincer 2008 [46] TUR | AS | mNY | 111 8:103 | SC | Upper-middle | NA | NA | 14.4 (14.2) vs. 5.3 (5.7) p = 0.06 |
| Garrido- Cumbrera 2021 [47] EUR | ax-SpA | Self- reported ASAS | 2846 1746: 1100 | MC | High | 26.4 (10.7) vs. 27.0 (11.8) p = 0.3 | 34.4 (10.9) vs. 32.6 (12.2) p < 0.001 | 8.2 (8.9) vs. 6.1 (7.4) p < 0.001 |
| Hajialilo 2014 [24] IRN | AS | mNY | 60 7:53 | SC | Upper-middle | NR | NA | 8.0 (4.7) vs. 5.9 (3.3) p = 0.14 |
| Ibn Yacoub 2012 [48] MAR | AS | mNY | 130 43:87 | SC | Lower-middle | 28.8 (10.7) vs. 27.9 (11.1) p = 0.4 | NR | 4.8 (2.7) vs. 4.6 (3.1) p = 0.07 |
| Jovani 2018 [22] EUR | ax-SpA and AS | ASAS/ mNY/ ESSG/ Amor | 150 54:96 | SC | High | 31.2 vs. 30.9 p = 0.9 | 41.9 vs. 39.1 p = 0.2 | * 7.5 (11) vs. * 4 (11) p = 0.05 |
| Landi 2016 [49] SA | AS | mNY | 1072 255:817 | MC | Upper-middle | NR | NR | 7.8 (7.5) vs. 8.9 (8.2) p = 0.1 |
| Li 2019 [50] CHN | ax-SpA and AS | ASAS, mNY | 208 59:149 | SC | Upper-middle | NA | NR | * 12.5 (1.5, 56.0) vs. * 35.0 (5.5, 87.3) (months) p = 0.014 |
| Ma 2012 [51] CHN | AS | mNY | 150 36:114 | MC | Upper-middle | south: 26.6 (6.6) vs. 24.1 (6.9); north: 28.6 (9.4) Vs. 26.9 (10.9) | NR | south: 6.2 (6.4) vs. 6.6 (6.0); north: 4.1 (6.3) vs. 4.0 (5.2) |
| Marks 1983 [52] USA | AS | NY | 50 25:25 | SC | High | 23 vs. 22.2 | NR | 12.8 vs. 10.3 |
| Neuenschwander 2020 [53] EUR | ax-SpA | ASAS | 495 264:231 | SC | High | 28.7 (9.1) vs. 28.3 (8.4) p = 0.6 | NR | 6.0 (7.8) vs. 4.7 (7.6) p = 0.005 |
| Ogdie 2019 [54] USA | AS | Self reported mNY | 235 174:61 | MC | High | NR | NR | NR |
| Reed 2008 [55] AUS | AS | mNY | 126 35:91 | SC | High | NA | NA | 10.2 vs. 7.3 |
| Ringsdal 1989 [56] EUR | AS | Self-reported mNY | 179 48:131 | SC | High | NA | NR | 12.6 vs. 9.5 |
| Roussou 2011 [57] EUR | ax-SpA and AS | ASAS/ mNY /ESSG | 516 344:172 | SC | High | NR | 42.2 (13.8) vs. 41.4 (14.9) p = NS | 6.3 (7.2) vs. 5.6 (7.9) p = NS |
| Shahlaee A 2015 [58] IRN | AS | mNY | 320 67:253 | NT | Upper-middle | 24.3 (7.73) vs. 22.2 (7.14) p = 0.04 | 33 (9.5) vs. 30.2 (8.9) p = 0.025 | 8.8 (8.5) vs. 8 (7.2) p = 0.5 |
| Slobodin 2011 [59] ISR | ax-SpA and AS | ASAS/ mNY | 151 72:79 | MC | High | NR | 38.5 (12.3) vs. 35.6 (11.7) p = 0.13 | 5.7 (6.0) vs. 5.9 (6.4) p = 0.9 |
| Zink 2000 [60] EUR | AS | mNY | 8776 2729: 6047 | NT | High | 29.1 (11.4) vs. 27.9 (10.9) | NR | 6.1 (8.1) vs. 5.5 (7.5) |
| Study | HLA*B27, Clinical Presentation, Prevalence of Social Factors, and Their Influence on DD |
|---|---|
| Aggarwal et al. 2009 [37] |
|
| Almousa et al. 2023 [38] |
|
| Aloush et al. 2007 [39] |
|
| Atagunduz et al. 2010 [40] |
|
| Bandinelli et al. 2016 [42] |
|
| Blasco-Blasco et al. 2017 [43] |
|
| Bodur et al. 2012 [44] |
|
| Dincer et al. 2008 [46] |
|
| Garrido-Cumbrera et al. 2021 [47] |
|
| Hajialilo 2014 [24] |
|
| Jovani et al. 2018 [22] |
|
| Landi et al. 2016 [49] |
|
| Ma et al. 2012 [51] |
|
| Marks et al. 1983 [52] |
|
| Neuenschwander et al. 2020 [53] |
|
| Ogdie et al. 2019 [54] |
|
| Shahlaee et al. 2015 [58] |
|
| Slobodin et al. 2011 [59] |
|
| Zink et al. 2000 [60] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandinelli, F.; Martinelli-Consumi, B.; Manetti, M.; Vallecoccia, M.S. Sex Bias in Diagnostic Delay: Are Axial Spondyloarthritis and Ankylosing Spondylitis Still Phantom Diseases in Women? A Systematic Review and Meta-Analysis. J. Pers. Med. 2024, 14, 91. https://doi.org/10.3390/jpm14010091
Bandinelli F, Martinelli-Consumi B, Manetti M, Vallecoccia MS. Sex Bias in Diagnostic Delay: Are Axial Spondyloarthritis and Ankylosing Spondylitis Still Phantom Diseases in Women? A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2024; 14(1):91. https://doi.org/10.3390/jpm14010091
Chicago/Turabian StyleBandinelli, Francesca, Bianca Martinelli-Consumi, Mirko Manetti, and Maria Sole Vallecoccia. 2024. "Sex Bias in Diagnostic Delay: Are Axial Spondyloarthritis and Ankylosing Spondylitis Still Phantom Diseases in Women? A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 14, no. 1: 91. https://doi.org/10.3390/jpm14010091