NTRK Gene Fusions in Solid Tumors and TRK Inhibitors: A Systematic Review of Case Reports and Case Series
Abstract
:1. Introduction
1.1. Rationale
1.2. Objective
2. Methods
2.1. Protocol and Registration
2.2. Search
2.3. Data Charting Process
2.4. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
3.2. Synthesis of Results
3.3. Quality Assessment
4. Discussion
4.1. Summary of Evidence
4.2. Future Perspectives
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarantino, P.; Mazzarella, L.; Marra, A.; Trapani, D.; Curigliano, G. The Evolving Paradigm of Biomarker Actionability: Histology-Agnosticism as a Spectrum, Rather than a Binary Quality. Cancer Treat. Rev. 2021, 94, 102169. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Hierro, C.; Matos, I.; Martin-Liberal, J.; Ochoa de Olza, M.; Garralda, E. Agnostic-Histology Approval of New Drugs in Oncology: Are We Already There? Clin. Cancer Res. 2019, 25, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Pestana, R.C.; Sen, S.; Hobbs, B.P.; Hong, D.S. Histology-Agnostic Drug Development-Considering Issues beyond the Tissue. Nat. Rev. Clin. Oncol. 2020, 17, 555–568. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (accessed on 28 September 2022).
- FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (accessed on 28 September 2022).
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Advanced Solid Tumors. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors (accessed on 28 September 2022).
- FDA Grants Accelerated Approval to Dabrafenib in Combination with Trametinib for Unresectable or Metastatic Solid Tumors with BRAF V600E Mutation. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grantsaccelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (accessed on 28 September 2022).
- FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. Available online: https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions (accessed on 28 September 2022).
- FDA Approves Entrectinib for NTRK Solid Tumors and ROS-1 NSCLC. Available online: https://www.fda.gov/drugs/resourcesinformation-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (accessed on 28 September 2022).
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular Characterization of Cancers with NTRK Gene Fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying Patients with NTRK Fusion Cancer. Ann. Oncol. 2019, 30, viii16–viii22. [Google Scholar] [CrossRef] [Green Version]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular Profiling for Precision Cancer Therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- PRISMA—Transparent Reporting of Systematic Reviews and Meta-Analyses. Available online: http://prisma-statement.org/ (accessed on 28 September 2022).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landman, Y.; Ilouze, M.; Wein, S.; Neiman, V.; Yerushalmi, R.; Yakimov, M.; Ku, N.; Schrock, A.B.; Ali, S.; Peled, N. Rapid Response to Larotrectinib (LOXO-101) in an Adult Chemotherapy-Naive Patients with Advanced Triple-Negative Secretory Breast Cancer Expressing ETV6-NTRK3 Fusion. Clin. Breast Cancer 2018, 18, e267–e270. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.S.; Wong, M.; Mayoh, C.; Kumar, A.; Tsoli, M.; Mould, E.; Tyrrell, V.; Khuong-Quang, D.-A.; Pinese, M.; Gayevskiy, V.; et al. Brief Report: Potent Clinical and Radiological Response to Larotrectinib in TRK Fusion-Driven High-Grade Glioma. Br. J. Cancer 2018, 119, 693–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.D.; Vargas, A.C.; Bonar, F.; Maclean, F.; Kattampallil, J.; Stewart, C.; Sulaiman, B.; Santos, L.; Gill, A.J. NTRK-Rearranged Mesenchymal Tumours: Diagnostic Challenges, Morphological Patterns and Proposed Testing Algorithm. Pathology 2020, 52, 401–409. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Setinek, U.; Krenbek, D.; Fazekas, A.; Illini, O.; Weinlinger, C.; Draxler, H.; Marcher, M.; Valipour, A.; Müllauer, L.; et al. Rapid Clinical and Radiologic Responses with Larotrectinib Treatment in a Patient with TRK-Fusion-Positive Metastatic Lung Cancer. Clin. Lung Cancer 2020, 21, e49–e53. [Google Scholar] [CrossRef]
- Alharbi, M.; Mobark, N.A.; Balbaid, A.A.O.; Alanazi, F.A.; Aljabarat, W.A.R.; Bakhsh, E.A.; Ramkissoon, S.H.; Abedalthagafi, M. Regression of ETV6-NTRK3 Infantile Glioblastoma After First-Line Treatment with Larotrectinib. JCO Precis. Oncol. 2020, 4, PO.20.00017. [Google Scholar] [CrossRef]
- Mayr, L.; Guntner, A.S.; Madlener, S.; Schmook, M.T.; Peyrl, A.; Azizi, A.A.; Dieckmann, K.; Reisinger, D.; Stepien, N.M.; Schramm, K.; et al. Cerebrospinal Fluid Penetration and Combination Therapy of Entrectinib for Disseminated ROS1/NTRK-Fusion Positive Pediatric High-Grade Glioma. J. Pers. Med. 2020, 10, 290. [Google Scholar] [CrossRef]
- Walter, A.W.; Kandula, V.V.R.; Shah, N. Larotrectinib Imaging Response in Low-Grade Glioma. Pediatr. Blood Cancer 2020, 67, e28002. [Google Scholar] [CrossRef]
- Salame, H.; Mckey, R.; Ballout, M.; Saad, W. The First Reported Case of Neurotrophic Tyrosine Receptor Kinase Fusion-Positive Thymoma Treated Successfully with Entrectinib. Cureus 2021, 13, e20588. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Tian, Y.; Wang, H.; Yang, X. A Novel NCOR2-NTRK1 Fusion Detected in a Patient of Lung Adenocarcinoma and Response to Larotrectinib: A Case Report. BMC Pulm. Med. 2021, 21, 125. [Google Scholar] [CrossRef]
- Gupta, M.; Sherrow, C.; Krone, M.E.; Blais, E.M.; Pishvaian, M.J.; Petricoin, E.F.; Matrisian, L.M.; DeArbeloa, P.; Gregory, G.; Brown, A.; et al. Targeting the NTRK Fusion Gene in Pancreatic Acinar Cell Carcinoma: A Case Report and Review of the Literature. J. Natl. Compr. Canc. Netw. 2021, 19, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Percy, C.; Schubert, T.; Galant, C.; Kirchgesner, T.; Mazzeo, F. Larotrectinib in a NTRK-Rearranged Soft Tissue Sarcoma in the Neoadjuvant Setting: A Case Report. Clin. Case Rep. 2021, 9, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Munkhdelger, J.; Shimooka, T.; Koyama, Y.; Ikeda, S.; Mikami, Y.; Fukuoka, J.; Hori, T.; Bychkov, A. Basaloid Squamous Cell Carcinoma of the Uterine Cervix: Report of a Case with Molecular Analysis. Int. J. Surg. Pathol. 2021, 29, 770–774. [Google Scholar] [CrossRef]
- Pircher, M.; Briner, H.R.; Bonomo, M.; Horcic, M.; Petrausch, U.; Helbling, D.; Winder, T. Mixed Response and Mechanisms of Resistance to Larotrectinib in Metastatic Carcinoma Ex Pleomorphic Adenoma of the Parotid Harboring an NTRK2 Fusion: A Case Report. Medicine (Baltimore) 2021, 100, e24463. [Google Scholar] [CrossRef] [PubMed]
- Pitoia, F. Complete Response to Larotrectinib Treatment in a Patient with Papillary Thyroid Cancer Harboring an ETV6-NTRK3 Gene Fusion. Clin. Case Rep. 2021, 9, 1905–1912. [Google Scholar] [CrossRef]
- Shepherd, D.J.; Miller, T.E.; Forst, D.A.; Jones, P.; Nardi, V.; Martinez-Lage, M.; Stemmer-Rachamimov, A.; Gonzalez, R.G.; Iafrate, A.J.; Ritterhouse, L.L. Mosaicism for Rec.ceptor Tyrosine Kinase Activation in a Glioblastoma Involving Both PDGFRA Amplification and NTRK2 Fusion. Oncologist 2021, 26, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.; Greim, R.; Krebs, K.; Luebben, F.; Dimmler, A. Characterization of an ETV6-NTRK3 Rearrangement with Unusual, but Highly Significant FISH Signal Pattern in a Secretory Carcinoma of the Salivary Gland: A Case Report. Diagn. Pathol. 2021, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Birzu, C.; Bielle, F.; Goulas, C.; Savatovsky, J.; Karachi, C.; Idbaih, A. Dramatic Response of STRN-NTRK-Fused Malignant Glioneuronal Tumor to Larotrectinib in Adult. Neuro Oncol. 2021, 23, 1200–1202. [Google Scholar] [CrossRef]
- Corral Sánchez, M.D.; Galán Gómez, V.; Sastre Urgelles, A.; Plaza López de Sabando, D.; Rubio Aparicio, P.; Martínez Martínez, L.; Alonso Gamarra, E.; Pozo Kreilinger, J.J.; Regojo Zapata, R.M.; López Gutiérrez, J.C.; et al. Treatment of Infantile Fibrosarcoma Associated to an Abdominal Aortic Aneurysm with Larotrectinib: A Case Report. Pediatr. Hematol. Oncol. 2021, 38, 504–509. [Google Scholar] [CrossRef]
- Goh, X.N.; Seng, M.S.-F.; Loh, A.H.P.; Gupta, A.; Chang, K.T.E.; Iyer, P. Larotrectinib Followed by Selitrectinib in a Novel DCTN1-NTRK1 Fusion Undifferentiated Pleomorphic Sarcoma. J. Oncol. Pharm. Pract. 2021, 27, 485–489. [Google Scholar] [CrossRef]
- Carter-Febres, M.; Schneller, N.; Fair, D.; Solomon, D.; Perry, A.; Roy, A.; Linscott, L.; Alashari, M.; Kestle, J.R.; Bruggers, C.S. Adjuvant Maintenance Larotrectinib Therapy in 2 Children with NTRK Fusion-Positive High-Grade Cancers. J. Pediatr. Hematol. Oncol 2021, 43, e987–e990. [Google Scholar] [CrossRef] [PubMed]
- Slomovic, A.; Amaral, T.; Lobko, I.; Siegel, D.N.; Goldfisher, R.; Kessel, R.; Levy, C.F. Comment on: A Newborn with a Large NTRK Fusion Positive Infantile Fibrosarcoma Successfully Treated with Larotrectinib. Pediatr. Blood Cancer 2021, 68, e28953. [Google Scholar] [CrossRef] [PubMed]
- Waters, T.W.; Moore, S.A.; Sato, Y.; Dlouhy, B.J.; Sato, M. Refractory Infantile High-Grade Glioma Containing TRK-Fusion Responds to Larotrectinib. Pediatr. Blood Cancer 2021, 68, e28868. [Google Scholar] [CrossRef]
- Mangum, R.; Reuther, J.; Bertrand, K.C.; Chandramohan, R.; Kukreja, M.K.; Paulino, A.C.; Muzny, D.; Hu, J.; Gibbs, R.A.; Curry, D.J.; et al. Durable Response to Larotrectinib in a Child with Histologic Diagnosis of Recurrent Disseminated Ependymoma Discovered to Harbor an NTRK2 Fusion: The Impact of Integrated Genomic Profiling. JCO Precis. Oncol. 2021, 5, PO.20.00375. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Watanabe, T.; Saito, M.; Saito, K.; Suzuki, R.; Sano, H.; Natori, Y.; Sasaki, E.; Ueda, M.; Kamo, N.; et al. A Rare Case of Recurrent Ovarian Cancer with TPM3-NTRK1 Gene Rearrangement: A Case Report. Mol. Clin. Oncol. 2022, 16, 90. [Google Scholar] [CrossRef]
- Ernst, M.S.; Lysack, J.T.; Hyrcza, M.D.; Chandarana, S.P.; Hao, D. TRK Inhibition with Entrectinib in Metastatic Salivary Secretory Carcinoma (SC): A Case Report. Curr. Oncol. 2022, 29, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Recine, F.; De Vita, A.; Fausti, V.; Pieri, F.; Bongiovanni, A.; Franchini, E.; Casadei, R.; Falasconi, M.C.; Oboldi, D.; Matteucci, F.; et al. Case Report: Adult NTRK-Rearranged Spindle Cell Neoplasm: Early Tumor Shrinkage in a Case with Bone and Visceral Metastases Treated with Targeted Therapy. Front. Oncol. 2021, 11, 740676. [Google Scholar] [CrossRef] [PubMed]
- Bill, R.; Deschler, D.G.; Pittet, M.J.; Pai, S.I.; Sadow, P.M.; Park, J.C. Diagnostic Challenges and Successful Organ-Preserving Therapy in a Case of Secretory Carcinoma of Minor Salivary Glands. Cancer Rep. (Hoboken) 2022, 5, e1491. [Google Scholar] [CrossRef]
- Bargas, S.; Mc Leer, A.; Mondet, J.; Chabre, O.; Laramas, M. An Impressive Response with Larotrectinib in a Patient with a Papillary Thyroid Carcinoma Harboring an SQSTM1-NTRK1 Fusion. Eur. J. Endocrinol. 2022, 186, K5–K8. [Google Scholar] [CrossRef]
- Kasi, P.M.; Afghan, M.K.; Bellizzi, A.M.; Chan, C.H. Larotrectinib in Mismatch-Repair-Deficient TRK Fusion-Positive Metastatic Colon Cancer after Progression on Immunotherapy. Cureus 2022, 14, e26648. [Google Scholar] [CrossRef]
- Saliba, M.; Mohanty, A.S.; Ho, A.L.; Drilon, A.; Dogan, S. Secretory Carcinoma of the Thyroid in a 49-Year-Old Man Treated with Larotrectinib: Protracted Clinical Course of Disease Despite the High-Grade Histologic Features. Head Neck Pathol. 2022, 16, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Lapeña, L.M.; Caldas, M.C.S.; Ramírez, C.; Basilio, M.S.; Junco, P.T.; Rodríguez-Laguna, L.; Martínez-González, V.; Marín-Manzano, E.; Perez-Martinez, A.; Lopez-Gutierrez, J.C. Larotrectinib as an Effective Therapy in Congenital Infantile Fibrosarcoma: Report of Two Cases. Eur. J. Pediatr. Surg. Rep. 2022, 10, e76–e79. [Google Scholar] [CrossRef] [PubMed]
- Groussin, L.; Theodon, H.; Bessiene, L.; Bricaire, L.; Bonnet-Serrano, F.; Cochand-Priollet, B.; Leroy, K.; Garinet, S.; Pasmant, E.; Zerbit, J.; et al. Redifferentiating Effect of Larotrectinib in NTRK-Rearranged Advanced Radioactive-Iodine Refractory Thyroid Cancer. Thyroid 2022, 32, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Grogan, P.T.; Deming, D.A.; Helgager, J.; Ruszkiewicz, T.; Baskaya, M.K.; Howard, S.P.; Robins, H.I. Entrectinib Demonstrates Prolonged Efficacy in an Adult Case of Radiation-Refractory NTRK Fusion Glioblastoma. Neurooncol. Adv. 2022, 4, vdac046. [Google Scholar] [CrossRef]
- Kobayashi, H.; Makise, N.; Shinozaki-Ushiku, A.; Zhang, L.; Ishibashi, Y.; Ikegami, M.; Tsuda, Y.; Kohsaka, S.; Ushiku, T.; Oda, K.; et al. Dramatic Response to Entrectinib in a Patient with Malignant Peripheral Nerve Sheath Tumor Harboring Novel SNRNP70-NTRK3 Fusion Gene. Genes Chromosom. Cancer 2022. [Google Scholar] [CrossRef]
- König, D.; Hench, J.; Frank, S.; Dima, L.; Bratic Hench, I.; Läubli, H. Larotrectinib Response in NTRK3 Fusion-Driven Diffuse High-Grade Glioma. Pharmacology 2022, 107, 433–438. [Google Scholar] [CrossRef]
- Olsen, M.R.; Denu, R.A.; Lyon, J.B.; Gulliver, J.M.; Capitini, C.M.; DeSantes, K.B. Undifferentiated and Unresectable Sarcoma with NTRK3-Fusion in a Pediatric Patient Treated with Larotrectinib and Proton Beam Radiotherapy. J. Pediatr. Hematol. Oncol. 2022, 44, e770–e774. [Google Scholar] [CrossRef]
- Mançano, B.M.; Dos Reis, M.B.; Moreno, D.A.; de Paula, F.E.; de Almeida Junior, C.R.; Cavalcante, C.E.B.; Zanon, M.F.; Santana, I.V.V.; Matsushita, M.; de, M.; et al. A Unique Case Report of Infant-Type Hemispheric Glioma (Gliosarcoma Subtype) with TPR-NTRK1 Fusion Treated with Larotrectinib. Pathobiology 2022, 89, 178–185. [Google Scholar] [CrossRef]
- Di Ruscio, V.; Carai, A.; Del Baldo, G.; Vinci, M.; Cacchione, A.; Miele, E.; Rossi, S.; Antonelli, M.; Barresi, S.; Caulo, M.; et al. Molecular Landscape in Infant High-Grade Gliomas: A Single Center Experience. Diagnostics 2022, 12, 372. [Google Scholar] [CrossRef]
- Kurozumi, K.; Fujii, K.; Washio, K.; Ishida, J.; Otani, Y.; Sudo, T.; Tahara, M.; Ichimura, K.; Ennishi, D.; Date, I. Response to Entrectinib in a Malignant Glioneuronal Tumor with ARHGEF2-NTRK Fusion. Neurooncol. Adv. 2022, 4, vdac094. [Google Scholar] [CrossRef]
- Iannantuono, G.M.; Torino, F.; Rosenfeld, R.; Guerriero, S.; Carlucci, M.; Sganga, S.; Capotondi, B.; Riondino, S.; Roselli, M. The Role of Histology-Agnostic Drugs in the Treatment of Metastatic Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 8535. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.-M.; Nawaz, K.; Webster, R.M. Tumour-Agnostic Therapies. Nat. Rev. Drug Discov. 2020, 19, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Sartore-Bianchi, A.; Siena, S. NTRK Gene Fusions as Novel Targets of Cancer Therapy across Multiple Tumour Types. ESMO Open 2016, 1, e000023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wei, Y.; Zhang, H.; Jiang, J.; Zhang, P.; Chu, Q. NTRK Fusion in Non-Small Cell Lung Cancer: Diagnosis, Therapy, and TRK Inhibitor Resistance. Front. Oncol. 2022, 12, 864666. [Google Scholar] [CrossRef]
- Vaishnavi, A.; Capelletti, M.; Le, A.T.; Kako, S.; Butaney, M.; Ercan, D.; Mahale, S.; Davies, K.D.; Aisner, D.L.; Pilling, A.B.; et al. Oncogenic and Drug-Sensitive NTRK1 Rearrangements in Lung Cancer. Nat. Med. 2013, 19, 1469–1472. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.Y.; Goldman, D.A.; Hechtman, J.F.; Benayed, R.; Schram, A.M.; Cocco, E.; Shifman, S.; Gong, Y.; Kundra, R.; Solomon, J.P.; et al. TRK Fusions Are Enriched in Cancers with Uncommon Histologies and the Absence of Canonical Driver Mutations. Clin. Cancer Res. 2020, 26, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, A.; Zhang, W.; Phillip Strauss, U.; Fellous, M.; Korei, M.; Keating, K. A Systematic Review and Meta-Analysis of Neurotrophic Tyrosine Receptor Kinase Gene Fusion Frequencies in Solid Tumors. Ther. Adv. Med. Oncol. 2020, 12, 1758835920975613. [Google Scholar] [CrossRef]
- Westphalen, C.B.; Krebs, M.G.; Le Tourneau, C.; Sokol, E.S.; Maund, S.L.; Wilson, T.R.; Jin, D.X.; Newberg, J.Y.; Fabrizio, D.; Veronese, L.; et al. Genomic Context of NTRK1/2/3 Fusion-Positive Tumours from a Large Real-World Population. NPJ Precis. Oncol. 2021, 5, 69. [Google Scholar] [CrossRef]
- Hechtman, J.F. NTRK Insights: Best Practices for Pathologists. Mod. Pathol. 2022, 35, 298–305. [Google Scholar] [CrossRef]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M.; Hechtman, J.F.; Troiani, T.; López-Rios, F.; Douillard, J.-Y.; et al. ESMO Recommendations on the Standard Methods to Detect NTRK Fusions in Daily Practice and Clinical Research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Drilon, A.E.; Hong, D.S.; van Tilburg, C.M.; Doz, F.; Tan, D.S.W.; Kummar, S.; Lin, J.J.; McDermott, R.S.; Zwaan, C.M.; Norenberg, R.; et al. Long-Term Efficacy and Safety of Larotrectinib in a Pooled Analysis of Patients with Tropomyosin Receptor Kinase (TRK) Fusion Cancer. JCO 2022, 40, 3100. [Google Scholar] [CrossRef]
- Iannantuono, G.M.; Riondino, S.; Sganga, S.; Roselli, M.; Torino, F. Activity of ALK Inhibitors in Renal Cancer with ALK Alterations: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 3995. [Google Scholar] [CrossRef] [PubMed]
- Gambella, A.; Senetta, R.; Collemi, G.; Vallero, S.G.; Monticelli, M.; Cofano, F.; Zeppa, P.; Garbossa, D.; Pellerino, A.; Rudà, R.; et al. NTRK Fusions in Central Nervous System Tumors: A Rare, but Worthy Target. Int. J. Mol. Sci. 2020, 21, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Central Nervous System Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 28 September 2022).
- Doz, F.; van Tilburg, C.M.; Geoerger, B.; Højgaard, M.; Øra, I.; Boni, V.; Capra, M.; Chisholm, J.; Chung, H.C.; DuBois, S.G.; et al. Efficacy and Safety of Larotrectinib in TRK Fusion-Positive Primary Central Nervous System Tumors. Neuro Oncol. 2022, 24, 997–1007. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [Green Version]
- Awada, A.; Berghmans, T.; Clement, P.M.; Cuppens, K.; De Wilde, B.; Machiels, J.-P.; Pauwels, P.; Peeters, M.; Rottey, S.; Van Cutsem, E. Belgian Expert Consensus for Tumor-Agnostic Treatment of NTRK Gene Fusion-Driven Solid Tumors with Larotrectinib. Crit. Rev. Oncol. Hematol. 2022, 169, 103564. [Google Scholar] [CrossRef]
- Registry for Molecular Testing, Treatment and Outcome of Patients with Solid Tumors Harboring a NTRK Gene Fusion (REALTRK). Available online: https://clinicaltrials.gov/ct2/show/NCT04557813 (accessed on 28 September 2022).
- Real Word European Registry of NTRK Fusions and Other Rare Actionable Fusions (TRacKING) (TRacKING). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04921553 (accessed on 28 September 2022).
- Smith, C.M.; Gilbert, E.B.; Riordan, P.A.; Helmke, N.; von Isenburg, M.; Kincaid, B.R.; Shirey, K.G. COVID-19-Associated Psychosis: A Systematic Review of Case Reports. Gen. Hosp. Psychiatry 2021, 73, 84–100. [Google Scholar] [CrossRef]
- Iannantuono, G.M.; Strigari, L.; Roselli, M.; Torino, F. A Scoping Review on the “Burned out” or “Burnt out” Testicular Cancer: When a Rare Phenomenon Deserves More Attention. Crit. Rev. Oncol. Hematol. 2021, 165, 103452. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, E.A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]



| Publication | Patients’ Characteristics | Treatments and Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Journal of Publication (Year) | Type of Evidence | Age (Sex) | Tumor Type | Histology | Sites of Metastases | NTRK Gene Fusion | Diagnosis | Previous Treatments | NTRK Inhibitor (Line of Therapy) | Best Radiological Response (Duration of Response) | Outcome |
| Landman et al. [19] | Clin Breast Cancer (2018) | CR | 37 (F) | BC | Secretory breast carcinoma | Bone, lung, lymph nodes, peritoneum, pleura | ETV6-NTRK3 | NGS | Surg-RT | Laro (1) | PR (6 *) | AwD |
| Ziegler et al. [20] | Br J Cancer (2018) | CR | 3 (F) | CNS | High-grade glioma | Loco-regional recurrence | ETV6–NTRK3 | NGS | Surg-ChT-RT | Laro (2) | PR (9 *) | AwD |
| Wong et al. [21] | Pathology (2020) | CS | 65 (F) | STS | Fibrosarcoma | Adrenal gland, kidney, liver, pancreas | ETV6-NTRK3 | FISH-IHC-NGS | Surg-RT | Entre (1) | PR (1 *) | AwD |
| Hochmair et al. [22] | Clin lung cancer (2020) | CR | 30 (F) | LC | Adenocarcinoma | Bone | TPM3-NTRK1 | IHC-NGS | Cht | Laro (2) | PR (4 *) | AwD |
| Alharbi et al. [23] | JCO Precis Oncol (2020) | CR | 2 (F) | CNS | High-grade glioma | Loco-regional recurrence | ETV6-NTRK3 | NGS | Surg | Laro (1) | PR (6 *) | AwD |
| Mayr et al. [24] | J Pers Med (2020) | CS | 9 (NA) | STS | Gliosarcoma | Bone, leptomeninges | EML4-NTRK3 | NGS | Surg-ChT-RT | Entre (3) | PR (5) | DoD |
| Walter et al. [25] | Pediatr Blood Cancer (2020) | CR | 6 (NA) | CNS | Low-Grade Glioma | No distant metastases | NACC2-NTRK | NA | ChT-TargT | Laro (5) | PR (NA) | NA |
| Salame et al. [26] | Cureus (2021) | CR | 50 (M) | Thymus | Thymoma | Pleura | EIF4B-NTRK3 | NGS | ChT | Entre (2) | PR (10 *) | AwD |
| Zhang et al. [27] | BMC Pulm Med (2021) | CR | 60 (F) | LC | Adenocarcinoma | Lung, pleura | NCOR2-NTRK1 | IHC- NGS | Surg-ICI | Laro (3) | PR (15 *) | AwD |
| Gupta et al. [28] | J Natl Compr Canc Netw (2021) | CR | 81 (M) | PC | Pancreatic acinar cell carcinoma | Liver, lymph nodes | SEL1L-NTRK1 | NGS | Surg-Cht | Laro (2) | PR (13 *) | AwD |
| Percy et al. [29] | Clin Case Rep (2021) | CR | 30 (M) | STS | Spindle cell sarcoma | No distant metastases | SPECC1L-NTRK | IHC-NGS | None | Laro (Neoadj) | PR (8 #) | AwD |
| Munkhdelger et al. [30] | Int J Surg Pathol (2021) | CR | 72 (F) | CC | Basaloid squamous cell carcinoma | Lung | DLG2-NTRK2 | NGS | Surg | Laro (1) | PR (NA) | NA |
| Pircher et al. [31] | Medicine (Baltimore) (2021) | CR | 63 (M) | SG | Carcinoma ex pleomorphic adenoma | Lung, lymph nodes | ZCCHC7-NTRK2 | NGS | Surg-RT | Laro (1) | SD | AwD |
| Pitoia et al. [32] | Clin Case Report (2021) | CR | 56 (F) | TT | Papillary | Adrenal gland, bone, brain, liver, lymph nodes, lung, pleura, soft tissue | ETV6-NTRK3 | NGS | RAI-TargT | Laro (3) | CR (11 *) | AwD |
| Shepherd et al. [33] | Oncologist (2021) | CR | 26 (M) | CNS | Glioblastoma | Loco-regional recurrence | KANK1-NTRK2 | NGS-FISH | CT/RT | Laro-Entre (2) | PR (3.5 §) | DoD |
| Wagner et al. [34] | Diagn Pathol (2021) | CR | 38 (M) | SG | Mammary analogue secretory carcinoma | Bone, lungs | ETV6-NTRK3 | IHC-FISH-NGS | Surg-Cht -CT/RT | Laro (1) | PR (8 *) | AwD |
| Boyer et al. [35] | Neuro Oncol (2021) | CR | 53 (M) | CNS | High-grade glioma | Loco-regional recurrence | STRN1-NTRK2 | NGS | Surg-CT/RT | Laro (2) | CR (11 *) | AwD |
| Corral Sánchez et al. [36] | Pediatr Hematol Oncol (2021) | CR | <1 (F) | STS | Infantile fibrosarcoma | No distant metastases | ETV6-NTRK3 | FISH | None | Laro (1) | CR (14 *) | AwD |
| Goh et al. [37] | J Oncol Pharm Pract (2021) | CR | 14 (M) | STS | Non-rhabdomyosarcoma soft tissue sarcoma | Soft tissues | DCTN1–NTRK1 | IHC-NGS | ChT-RT-Surg | Laro (2) | PR (6) | DoD |
| Carter-Febres et al. [38] | J Pediatr Hematol Oncol (2021) | CS | 2 (F) | STS | Undifferentiated embryonal sarcoma | No distant metastases | ETV6-NTRK3 | NGS | ChT-Surg | Laro (Adj) | CR (12 *) | AwD |
| 3 (M) | CNS | High-grade glioma | No distant metastases | NACC2-NTRK2 | NGS | Surg-CT/RT | Laro (Adj) | CR (15 *) | AwD | |||
| Slomovic et al. [39] | Pediatr Blood Cancer (2021) | CR | <1 (M) | STS | Infantile fibrosarcoma | No distant metastases | ETV6-NTRK | NA | ChT | Laro (2) | PR (14 *) | AwD |
| Waters et al. [40] | Pediatr Blood Cancer (2021) | CR | 2 (M) | CNS | Glioma | Loco-regional recurrence | EML4-NTRK3 | NA | Surg-ChT | Laro (2) | PR (12 *) | AwD |
| Mangum et al. [41] | JCO Precis Oncol (2021) | CR | 6 (M) | CNS | Ependymoma | Loco-regional recurrence, leptomeninges | KANK1-NTRK2 | NGS | Surg-RT | Laro (1) | PR (10 *) | AwD |
| Endo et al. [42] | Mol Clin Oncol (2022) | CR | 56 (F) | OC | High-Grade Serous Carcinoma | Lymph nodes, peritoneum, pleura, liver | TPM3-NTRK1 | NGS | ChT-Surg-TargT | Entre (6) | PD | DoD |
| Ernst et al. [43] | Curr Oncol (2022) | CR | 59 (M) | SG | Mammary analogue secretory carcinoma | Loco-regional recurrence, lung | ETV6-NTRK3 | FISH-NGS | Surg | Entre (1) | PR (49 *) | AwD |
| Recine et al. [44] | Front Oncol (2022) | CR | 14 (M) | STS | Dermatofibrosarcoma | Bone, kidney, liver, lung, soft tissue | TPM4-NTRK1 | NGS | Surg-RT-TargT | Laro (2) | PR (23 *) | AwD |
| Bill et al. [45] | Cancer Rep (Hoboken) (2022) | CR | 56 (F) | SG | Mammary analogue secretory carcinoma | Lymph nodes | ETV6-NTRK3 | IHC-NGS | Surg-CT/RT | Laro (2) | CR (13 *) | AwD |
| Bargas et al. [46] | Eur J Endocrinol (2022) | CR | 50 (F) | TT | Papillary | Lung, ovary, mediastinum, lymph node | SQSTM1-NTRK1 | NGS-FISH | Surg-RAI-TargT | Laro (3) | PR (18 *) | AwD |
| Kasi et al. [47] | Cureus (2022) | CR | 43 (F) | CoC | Adenocarcinoma | Lymph nodes, peritoneum | TPR-NTRK1 | NGS-IHC | Surg-ICI | Laro (2) | PR (3 †) | AwD |
| Saliba et al. [48] | Head Neck Pathol (2022) | CR | 49 (M) | TT | Secretory carcinoma | Loco-regional recurrence, lymph nodes, lung | ETV6-NTRK3 | NGS | Surg | Laro (1) | PR (18) | DoD |
| Lapeña et al. [49] | European J Pediatr Surg Rep (2022) | CS | <1 (F) | STS | Infantile fibrosarcoma | No distant metastases | ETV6-NTRK3 | NA | None | Laro (1) | CR (14 *) | AwD |
| <1 (M) | STS | Infantile fibrosarcoma | No distant metastases | ETV6-NTRK3 | NA | None | Laro (1) | CR (6 *) | AwD | |||
| Groussin et al. [50] | Thyroid (2022) | CS | 65 (F) | TT | Papillary | Bone, liver, lymph nodes, lung | EML4-NTRK3 | NGS | RAI-TargT | Laro (3) | PR (NA) | NA |
| 48 (F) | TT | Papillary | Lymph nodes, lung | ETV6-NTRK3 | NGS | RAI-TargT | Laro (3) | PR (NA) | NA | |||
| 70 (F) | TT | Oxyphilic cell papillary | Brain, bone, lymph nodes, liver, lung, pancreas, soft Tissue | TPM3-NTRK1 | NGS | Surg | Laro (1) | PR (NA) | NA | |||
| Grogan et al. [51] | Neurooncol Adv (2022) | CR | 67 (M) | CNS | Glioblastoma | Loco-regional recurrence | BCR-NTRK2 | NGS | Surg-RT | Entre (1) | PR (15) | NA |
| Kobayashi et al. [52] | Genes Chromosomes Cancer (2022) | CR | 57 (M) | STS | Malignant peripheral nerve sheath tumors | Lymph nodes, lung | SNRNP70-NTRK3 | FISH-IHC-NGS | Surg-RT-ChT- TargT | Entre (4) | PR (11) | NA |
| König et al. [53] | Pharmacology (2022) | CR | 80 (F) | CNS | High-grade glioma | No distant metastases | ARHGEF7-NTRK3 | NGS | RT | Laro (1) | PR (4.5) | AwD |
| Olsen et al. [54] | J Pediatr Hematol Oncol (2022) | CR | 6 (F) | STS | High-grade spindle cell sarcoma | Bone | NTRK3 gene rearrangement | IHC-FISH | ChT-RT | Laro (3) | PR (22) | AwD |
| Mançano et al. [55] | Pathobiology (2022) | CR | <1 (M) | STS | Gliosarcoma | Loco-regional recurrence | TPR-NTRK1 | FISH-IHC-NGS | Surg-ChT | Laro (2) | PR (8 *) | AwD |
| Di Ruscio et al. [56] | Diagnostics (2022) | CS | 1 (NA) | CNS | High-grade glioma | Loco-regional recurrence | ETV6-NTRK3 | NGS | Surg-ChT-TargT | Laro (2) | CR (24 *) | AwD |
| 1 (F) | CNS | High-grade glioma | Loco-regional recurrence | MEF2D-NTRK1 | NGS | Surg-ChT | Laro (2) | PR (4 *) | AwD | |||
| Included Publications | Sites of Metastases—n (%) | Diagnosis—n (%) † | |||
|---|---|---|---|---|---|
| Number of CR | 32 | LR recurrence | 13 (38.2%) | NGS | 36 (83.7%) |
| Number of CS | 6 | Lymph nodes | 13 (38.2%) | IHC | 12 (27.9%) |
| Year of publication (Range) | 2018–2022 | Lung | 13 (38.2%) | FISH | 8 (18.6%) |
| Demographics—n (%) | Bone | 9 (26.5%) | RT-PCR | 0 (0%) | |
| Liver | 7 (20.6%) | NTRKi [Drug]—n (%) | |||
| Number of patients | 43 | Pleura | 5 (14.7%) | ||
| Median Age | 37 (<1–81) | Soft tissue | 5 (14.7%) | Larotrectinib | 35 (81.4%) |
| Adult–Children | 25 (58.1%)–18 (41.9%) | Brain | 3 (8.8%) | Entrectinib | 7 (16.3%) |
| Male–Female * | 19 (44.2%)–21 (48.8%) | Adrenal | 3 (8.8%) | Both | 1 (2.3%) |
| Tumor types—n (%) | Peritoneum | 3 (8.8%) | NTRKi [Line of therapy]—n (%) § | ||
| Mediastinum | 2 (5.9%) | ||||
| Soft tissue sarcoma | 13 (30.2%) | Kidney | 2 (5.9%) | First-line | 15 (34.9%) |
| CNS tumors | 12 (27.9%) | Leptomeninges | 2 (5.9%) | Second-line | 15 (34.9%) |
| Thyroid tumors | 6 (14%) | Pancreas | 2 (5.9%) | Third-line | 7 (16.3%) |
| Salivary gland tumors | 4 (9.3%) | Ovarian | 1 (2.9%) | Subsequent lines | 3 (6.9%) |
| Lung tumor | 2 (4.8%) | NTRK gene fusion partner—n (%) ** | NTRKi [Best radiological response]—n (%) | ||
| Breast cancer | 1 (2.3%) | ||||
| Colon cancer | 1 (2.3%) | ETV6 | 16 (37.2%) | Partial response | 32 (74.5%) |
| Ovarian cancer | 1 (2.3%) | TMP3 | 3 (7%) | Complete response | 9 (20.9%) |
| Pancreatic tumor | 1 (2.3%) | EML4 | 3 (7%) | Stable disease | 1 (2.3%) |
| Thymus | 1 (2.3%) | Site of NTRK gene fusion detection—n (%) # | Progressive disease | 1 (2.3%) | |
| Cervix cancer | 1 (2.3%) | NTRKi [Outcomes]—n (%) ## | |||
| Stage—n (%) | Primary tumor | 30 (69.7%) | |||
| Metastasis | 7 (16.3%) | Alive with disease | 31 (72.1%) | ||
| Metastatic | 34 (79.1%) | Dead of disease | 5 (11.6%) | ||
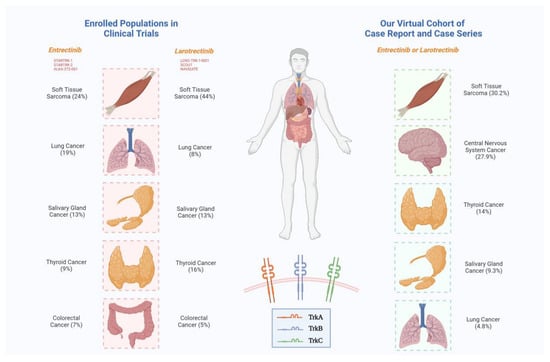
| Tumor Types | Larotrectinib (LOXO-TRK-14001; SCOUT; NAVIGATE) [68] | Entrectinib (STARTRK-1; STARTRK-2; ALKA-372-001) [15] | Larotrectinib + Entrectinib (Virtual Cohort of CR and CS) [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
|---|---|---|---|
| Appendix cancer | 1 (<1%) | - | - |
| Bone sarcoma | 2 (1%) | - | - |
| Breast cancer | 5 (3%) | 6 (11%) | 1 (2.3%) |
| Congenital mesoblastic nephroma | 1 (<1%) | - | - |
| Cholangiocarcinoma | 2 (1%) | 1 (2%) | - |
| Colorectal cancer | 8 (5%) * | 4 (7%) | 1 (2.3%) * |
| Cervical cancer | - | - | 1 (2.3%) |
| Endometrial cancer | - | 1 (2%) | - |
| Central nervous system tumor | - | - | 12 (27.9%) |
| Hepatocellular tumor | 1 (<1%) | - | - |
| Lung cancer | 12 (8%) | 10 (19%) ** | 2 (4.8%) |
| Melanoma | 7 (4%) | - | - |
| Neuroendocrine tumor | - | 3 (6%) | - |
| Ovarian cancer | - | 1 (2%) | 1 (2.3%) |
| Pancreas cancer | 2 (1%) | 3 (6%) | 1 (2.3%) |
| Prostate cancer | 1 (<1%) | - | - |
| Salivary gland tumor | 21 (13%) | 7 (13%) † | 4 (9.3%) |
| Soft tissue sarcoma | 69 (44%) # | 13 (24%) § | 13 (30.2%) |
| Thymoma | - | - | 1 (2.3%) |
| Thyroid cancer | 26 (16%) | 5 (9%) | 6 (14%) |
| Unknown primary | 1 (<1%) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannantuono, G.M.; Riondino, S.; Sganga, S.; Rosenfeld, R.; Guerriero, S.; Carlucci, M.; Capotondi, B.; Torino, F.; Roselli, M. NTRK Gene Fusions in Solid Tumors and TRK Inhibitors: A Systematic Review of Case Reports and Case Series. J. Pers. Med. 2022, 12, 1819. https://doi.org/10.3390/jpm12111819
Iannantuono GM, Riondino S, Sganga S, Rosenfeld R, Guerriero S, Carlucci M, Capotondi B, Torino F, Roselli M. NTRK Gene Fusions in Solid Tumors and TRK Inhibitors: A Systematic Review of Case Reports and Case Series. Journal of Personalized Medicine. 2022; 12(11):1819. https://doi.org/10.3390/jpm12111819
Chicago/Turabian StyleIannantuono, Giovanni Maria, Silvia Riondino, Stefano Sganga, Roberto Rosenfeld, Simona Guerriero, Manuela Carlucci, Barbara Capotondi, Francesco Torino, and Mario Roselli. 2022. "NTRK Gene Fusions in Solid Tumors and TRK Inhibitors: A Systematic Review of Case Reports and Case Series" Journal of Personalized Medicine 12, no. 11: 1819. https://doi.org/10.3390/jpm12111819