Percutaneous Coronary Angioplasty in Patients with Cancer: Clinical Challenges and Management Strategies
Abstract
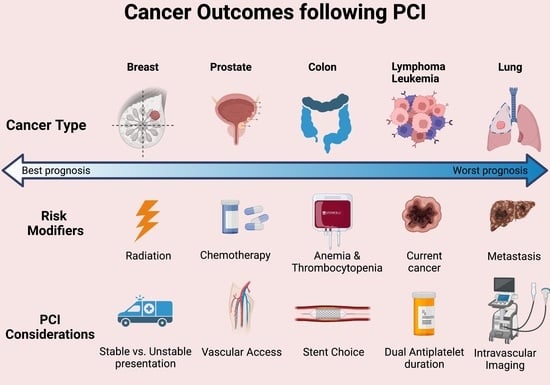
:1. Introduction
2. PCI in Cancer: Specific Challenges
3. PCI Outcomes in Cancer Patients
4. Multidisciplinary Approach
5. Vascular Access
6. Pharmacology
7. Thrombocytopenia
8. Intravascular Physiological Assessment
9. Stent Choice
10. Intracoronary Imaging
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AMI | Acute myocardial infarction |
| AML | Acute myeloid leukaemia |
| BMS | Bare metal stent |
| CAD | Coronary artery disease |
| CVD | Cardiovascular disease |
| DCS | Drug-coated stent |
| DAPT | Dual antiplatelet therapy |
| DES | Drug-eluting stent |
| FFR | Fractional flow reserve |
| HBR | High bleeding risk |
| ISR | In-stent restenosis |
| MACE | Major adverse cardiac events |
| MLA | Minimum lumen areas |
| OCT | Optical coherence tomography |
| OR | Odds ratio |
| PCI | Percutaneous coronary intervention |
| RR | Relative risk |
| ST | Stent thrombosis |
| TLR | Target lesion revascularisation |
| TVR | Target vessel revascularisation |
References
- Office of Cancer Survivorship. Statistics and Graphs; Office of Cancer Survivorship: Rockville, MD, USA, 2022. [Google Scholar]
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021; American Cancer Society: Kennesaw, GA, USA, 2019. [Google Scholar]
- Mamas, M.A.; Brown, S.-A.; Sun, L.Y. Coronary Artery Disease in Patients With Cancer: It’s Always the Small Pieces That Make the Bigger Picture. Mayo Clin. Proc. 2020, 95, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; Kelly, S.; Zaorsky, N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; Kentepozidis, N.; Kotsakis, A.; Kapiris, I. Cancer therapy and cardiovascular risk: Focus on bevacizumab. Cancer Manag. Res. 2015, 7, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Arunprasath, P.; Gobu, P.; Dubashi, B.; Satheesh, S.; Balachander, J. Rituximab induced myocardial infarction: A fatal drug reaction. J. Cancer Res. Ther. 2011, 7, 346–348. [Google Scholar] [CrossRef]
- Yeh, E.T.; Tong, A.T.; Lenihan, D.J.; Yusuf, S.W.; Swafford, J.; Champion, C.; Durand, J.-B.; Gibbs, H.; Zafarmand, A.A.; Ewer, M.S. Cardiovascular Complications of Cancer Therapy. Circulation 2004, 109, 3122–3131. [Google Scholar] [CrossRef]
- Veinot, J.P.; Edwards, W.D. Pathology of radiation-induced heart disease: A surgical and autopsy study of 27 cases. Hum. Pathol. 1996, 27, 766–773. [Google Scholar] [CrossRef]
- Mamas, M.A.; Fath-Ordoubadi, F.; Danzi, G.B.; Spaepen, E.; Kwok, C.S.; Buchan, I.; Peek, N.; de Belder, M.A.; Ludman, P.F.; Paunovic, D.; et al. Prevalence and Impact of Co-morbidity Burden as Defined by the Charlson Co-morbidity Index on 30-Day and 1- and 5-Year Outcomes After Coronary Stent Implantation (from the Nobori-2 Study). Am. J. Cardiol. 2015, 116, 364–371. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Shiomi, H.; Morimoto, T.; Watanabe, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; Ono, K.; Shizuta, S.; et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 200–207. [Google Scholar] [CrossRef]
- Hess, C.N.; Roe, M.T.; Clare, R.M.; Chiswell, K.; Kelly, J.; Tcheng, J.E.; Hagstrom, E.; James, S.K.; Khouri, M.G.; Hirsch, B.R.; et al. Relationship Between Cancer and Cardiovascular Outcomes Following Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2015, 4, e001779. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Potts, J.; Mohamed, M.; Parwani, P.; Swamy, P.; Lopez-Mattei, J.C.; Rashid, M.; Kwok, C.S.; Fischman, D.L.; Vassiliou, V.S.; et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur. Heart J. 2020, 41, 2183–2193. [Google Scholar] [CrossRef]
- Iannaccone, M.; D’Ascenzo, F.; Vadalà, P.; Wilton, S.B.; Noussan, P.; Colombo, F.; Roubín, S.R.; Abu Assi, E.; González-Juanatey, J.R.; Henriques, J.P.S.; et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: A BleeMACS substudy. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 631–638. [Google Scholar] [CrossRef]
- Velders, M.A.; Boden, H.; Hofma, S.H.; Osanto, S.; van der Hoeven, B.L.; Heestermans, A.A.; Cannegieter, S.C.; Jukema, J.W.; Umans, V.A.; Schalij, M.J.; et al. Outcome After ST Elevation Myocardial Infarction in Patients With Cancer Treated With Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2013, 112, 1867–1872. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.; Panageas, K.S.; DeAngelis, L.M. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef]
- Johnstone, C.; Rich, S.E. Bleeding in cancer patients and its treatment: A review. Ann. Palliat. Med. 2018, 7, 265–273. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef]
- Apter, S.; Shemesh, J.; Raanani, P.; Portnoy, O.; Thaler, M.; Zissin, R.; Ezra, D.; Rozenman, J.; Pfeffer, R.; Hertz, M. Cardiovascular calcifications after radiation therapy for Hodgkin lymphoma: Computed tomography detection and clinical correlation. Coron. Artery Dis. 2006, 17, 145–151. [Google Scholar] [CrossRef]
- Ali, Z.A.; Nef, H.; Escaned, J.; Werner, N.; Banning, A.; Hill, J.M.; De Bruyne, B.; Montorfano, M.; Lefevre, T.; Stone, G.W.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses. Circ. Cardiovasc. Interv. 2019, 12, e008434. [Google Scholar] [CrossRef]
- Mori, S.; Yasuda, S.; Kataoka, Y.; Morii, I.; Kawamura, A.; Miyazaki, S. Significant Association of Coronary Artery Calcification in Stent Delivery Route With Restenosis After Sirolimus-Eluting Stent Implantation. Circ. J. 2009, 73, 1856–1863. [Google Scholar] [CrossRef]
- Kini, A.S.; Vengrenyuk, Y.; Pena, J.; Motoyama, S.; Feig, J.E.; Meelu, O.; Rajamanickam, A.; Bhat, A.M.; Panwar, S.; Baber, U.; et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter. Cardiovasc. Interv. 2015, 86, 1024–1032. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Zhu, L.-L.; Bourantas, C.V.; Iqbal, J.; Dong, S.-J.; Campos, C.M.; Li, M.-H.; Ye, F.; Tian, N.-L.; Garcia-Garcia, H.M.; et al. The impact of everolimus versus other rapamycin derivative-eluting stents on clinical outcomes in patients with coronary artery disease: A meta-analysis of 16 randomized trials. J. Cardiol. 2014, 64, 185–193. [Google Scholar] [CrossRef]
- Potts, J.E.; Iliescu, C.; Lopez-Mattei, J.; Martinez, S.C.; Holmvang, L.; Ludman, P.; De Belder, M.; Kwok, C.S.; Rashid, M.; Fischman, D.L.; et al. Percutaneous coronary intervention in cancer patients: A report of the prevalence and outcomes in the United States. Eur. Heart J. 2019, 40, 1790–1800. [Google Scholar] [CrossRef]
- Kwok, C.S.; Wong, C.W.; Kontopantelis, E.; Barac, A.; Brown, S.-A.; Velagapudi, P.; Hilliard, A.; Bharadwaj, A.S.; Alraies, M.C.; Mohamed, M.; et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur. Heart J. 2021, 42, 1019–1034. [Google Scholar] [CrossRef]
- Blann, A.D.; Dunmore, S. Arterial and Venous Thrombosis in Cancer Patients. Cardiol. Res. Pract. 2011, 2011, 394740. [Google Scholar] [CrossRef]
- Anderson, S.G.; Ratib, K.; Myint, P.K.; Keavney, B.; Kwok, C.S.; Zaman, A.; Dm, B.B.K.; de Belder, M.A.; Nolan, J.; Mamas, M.A.; et al. Impact of age on access site-related outcomes in 469,983 percutaneous coronary intervention procedures: Insights from the British Cardiovascular Intervention Society. Catheter. Cardiovasc. Interv. 2015, 86, 965–972. [Google Scholar] [CrossRef]
- Quintana, R.A.; Monlezun, D.J.; Davogustto, G.; Saenz, H.R.; Lozano-Ruiz, F.; Sueta, D.; Tsujita, K.; Landes, U.; Denktas, A.E.; Alam, M.; et al. Outcomes following percutaneous coronary intervention in patients with cancer. Int. J. Cardiol. 2020, 300, 106–112. [Google Scholar] [CrossRef]
- Potts, J.; Mohamed, M.O.; Mattei, J.C.L.; Iliescu, C.A.; Konopleva, M.; Rashid, M.; Bagur, R.; Mamas, M.A. Percutaneous coronary intervention and in-hospital outcomes in patients with leukemia: A nationwide analysis. Catheter. Cardiovasc. Interv. 2020, 96, 53–63. [Google Scholar] [CrossRef]
- Pothineni, N.V.; Shah, N.N.; Rochlani, Y.; Saad, M.; Kovelamudi, S.; Marmagkiolis, K.; Bhatti, S.; Cilingiroglu, M.; Aronow, W.S.; Hakeem, A. Temporal trends and outcomes of acute myocardial infarction in patients with cancer. Ann. Transl. Med. 2017, 5, 482. [Google Scholar] [CrossRef] [PubMed]
- Shivaraju, A.; Patel, V.; Fonarow, G.; Xie, H.; Shroff, A.R.; Vidovich, M.I. Temporal trends in gastrointestinal bleeding associated with percutaneous coronary intervention: Analysis of the 1998-2006 Nationwide Inpatient Sample (NIS) database. Am. Heart J. 2011, 162, 1062–1068.e5. [Google Scholar] [CrossRef] [PubMed]
- Van Werkum, J.W.; Heestermans, A.A.; Zomer, A.C.; Kelder, J.C.; Suttorp, M.J.; Rensing, B.J.; Kollen, J.J.; Brueren, B.R.G.; Dambrink, J.-H.E.; Berg, J.M.; et al. Predictors of Coronary Stent Thrombosis: The Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 2009, 53, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Sueta, D.; Yamamoto, E.; Takashio, S.; Arima, Y.; Araki, S.; Yamanaga, K.; Ishii, M.; Sakamoto, K.; Kanazawa, H.; et al. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: A Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 290–300. [Google Scholar] [CrossRef]
- Thomason, N.; Monlezun, D.J.; Javaid, A.; Filipescu, A.; Koutroumpakis, E.; Shobayo, F.; Kim, P.; Lopez-Mattei, J.; Cilingiroglu, M.; Iliescu, G.; et al. Percutaneous Coronary Intervention in Patients With Gynecological Cancer: Machine Learning-Augmented Propensity Score Mortality and Cost Analysis for 383,760 Patients. Front Cardiovasc Med 2021, 8, 793877. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Van Spall, H.G.; Kontopantelis, E.; Alkhouli, M.; Barac, A.; Elgendy, I.Y.; Khan, S.U.; Kwok, C.S.K.; Shoaib, A.; Mamas, M.A. Effect of primary percutaneous coronary intervention on in-hospital outcomes among active cancer patients presenting with ST-elevation myocardial infarction: A propensity score matching analysis. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 829–839. [Google Scholar] [CrossRef]
- Velders, M.A.; Hagström, E.; James, S.K. Temporal Trends in the Prevalence of Cancer and Its Impact on Outcome in Patients With First Myocardial Infarction: A Nationwide Study. J. Am. Heart Assoc. 2020, 9, e014383. [Google Scholar] [CrossRef]
- Borovac, J.A.; Kwok, C.S.; Iliescu, C.; Lee, H.J.; Kim, P.Y.; Palaskas, N.L.; Zaman, A.; Butler, R.; Lopez-Mattei, J.C.; Mamas, M.A. Percutaneous Coronary Intervention and Outcomes in Patients With Lymphoma in the United States (Nationwide Inpatient Sample [NIS] Analysis). Am. J. Cardiol. 2019, 124, 1190–1197. [Google Scholar] [CrossRef]
- Landes, U.; Kornowski, R.; Bental, T.; Assali, A.; Vaknin-Assa, H.; Lev, E.; Iakobishvili, Z. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron. Artery Dis. 2017, 28. [Google Scholar] [CrossRef]
- Guddati, A.K.; Joy, P.S.; Kumar, G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J. Cancer Res. Clin. Oncol. 2016, 142, 471–479. [Google Scholar] [CrossRef]
- Wang, F.; Gulati, R.; Lennon, R.J.; Lewis, B.R.; Park, J.; Sandhu, G.S.; Wright, R.S.; Lerman, A.; Herrmann, J. Cancer History Portends Worse Acute and Long-term Noncardiac (but Not Cardiac) Mortality After Primary Percutaneous Coronary Intervention for Acute ST-Segment Elevation Myocardial Infarction. Mayo Clin. Proc. 2016, 91, 1680–1692. [Google Scholar] [CrossRef]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, E.; Poulin, M.F.; Goel, M.; Oyenusi, O.; Iliescu, C.; Okwuosa, T.M. PCI in patients with cancer. Card. Interv. Today 2019. Available online: https://citoday.com/articles/2019-jan-feb/pci-in-patients-with-cancer (accessed on 24 July 2022).
- Yusuf, S.W.; Daraban, N.; Abbasi, N.; Lei, X.; Durand, J.-B.; Daher, I.N. Treatment and Outcomes of Acute Coronary Syndrome in the Cancer Population. Clin. Cardiol. 2012, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, S.; Iwasaki, T.; Ishibashi, K.; Mitsuba, N.; Dohi, Y.; Kihara, Y. Comparison of treatment and outcome of acute myocardial infarction between cancer patients and non-cancer patients. Int. J. Cardiol. 2013, 167, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Hakim, D.A.; Dangas, G.D.; Caixeta, A.; Nikolsky, E.; Lansky, A.J.; Moses, J.W.; Claessen, B.; Sanidas, E.; White, H.D.; Ohman, E.M.; et al. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: Analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction. Am. Heart J. 2011, 161, 391–396. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Swamy, P.M.; Mamas, M.A. Outcomes of percutaneous coronary interventions in cancer patients. Expert Rev. Cardiovasc. Ther. 2020, 18, 25–32. [Google Scholar] [CrossRef]
- Ayoub, K.; Marji, M.; Ogunbayo, G.; Masri, A.; Abdel-Latif, A.; Ziada, K.; Vallurupalli, S. Impact of Chronic Thrombocytopenia on In-Hospital Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 1862–1868. [Google Scholar] [CrossRef]
- Kwok, C.S.; Tiong, D.; Pradhan, A.; Andreou, A.Y.; Nolan, J.; Bertrand, O.F.; Curzen, N.; Urban, P.; Myint, P.K.; Zaman, A.G.; et al. Meta-Analysis of the Prognostic Impact of Anemia in Patients Undergoing Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 118, 610–620. [Google Scholar] [CrossRef]
- Gragnano, F.; Heg, D.; Franzone, A.; McFadden, E.P.; Leonardi, S.; Piccolo, R.; Vranckx, P.; Branca, M.; Serruys, P.W.; Benit, E.; et al. PRECISE-DAPT score for bleeding risk prediction in patients on dual or single antiplatelet regimens: Insights from the GLOBAL LEADERS and GLASSY. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 28–38. [Google Scholar] [CrossRef]
- Valgimigli, M.; Frigoli, E.; Heg, D.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.-C.; Chevalier, B.; Smits, P.C.; et al. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N. Engl. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef]
- Valgimigli, M.; Smits, P.C.; Frigoli, E.; Bongiovanni, D.; Tijssen, J.; Hovasse, T.; Mafragi, A.; Ruifrok, W.T.; Karageorgiev, D.; Aminian, A.; et al. Duration of antiplatelet therapy after complex percutaneous coronary intervention in patients at high bleeding risk: A MASTER DAPT trial sub-analysis. Eur. Heart J. 2022, ehac284. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Jüni, P.; Hamm, C.; Steg, P.G.; Heg, D.; van Es, G.A.; McFadden, E.P.; Onuma, Y.; van Meijeren, C.; et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre. Lancet 2018, 392, 940–949. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2020, 382, 1208–1218. [Google Scholar] [CrossRef]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs. 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef]
- Ariotti, S.; Adamo, M.; Costa, F.; Patialiakas, A.; Briguori, C.; Thury, A.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I. Is Bare-Metal Stent Implantation Still Justifiable in High Bleeding Risk Patients Undergoing Percutaneous Coronary Intervention: A Pre-Specified Analysis From the ZEUS Trial. JACC Cardiovasc. Interv. 2016, 9, 426–436. [Google Scholar] [CrossRef]
- Valgimigli, M.; Cao, D.; Angiolillo, D.J.; Bangalore, S.; Bhatt, D.L.; Ge, J.; Hermiller, J.; Makkar, R.R.; Neumann, F.-J.; Saito, S.; et al. Duration of Dual Antiplatelet Therapy for Patients at High Bleeding Risk Undergoing PCI. J. Am. Coll. Cardiol. 2021, 78, 2060–2072. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European. Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Jeger, R.V.; Eccleshall, S.; Ahmad, W.A.W.; Ge, J.; Poerner, T.C.; Shin, E.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Uskela, S.; Eränen, J.; Mäntylä, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietila, M.; Minkkinen, M.J.; Palojoki, E.; et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): A single-blind, randomised, non-inferiority trial. Lancet 2019, 394, 230–239. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Iliescu, C.A.; Grines, C.L.; Herrmann, J.; Yang, E.H.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.P.; Leesar, M.A.; Marmagkiolis, K. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa interve. Catheter. Cardiovasc. Interv. 2016, 87, E202–E223. [Google Scholar] [CrossRef] [PubMed]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; Clemmensen, P.; et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Jeremias, A. FFR in 2017: Current Status in PCI Management. Am. Coll. Cardiol. 2017, 27. Available online: https://www.acc.org/latest-in-cardiology/articles/2017/05/25/08/34/ffr-in-2017-current-status-in-pci-management (accessed on 25 July 2022).
- Donisan, T.; Dayah, T.; Balanescu, D.V.; Karimzad, K.; Boone, D.L.; Lopez-Mattei, J.; Kim, P.Y.; Durand, J.-B.; Yang, E.H.; Herrmann, J.; et al. Clinical outcomes after fractional flow reserve-guided treatment of oncology patients. J. Clin. Oncol. 2018, 36, e22106. [Google Scholar] [CrossRef]
- Bagai, J.; Gasperetti, C. Cardio-Oncology for the Interventionalist—Part 2. SCAI 2019. Available online: https://scai.org/cardio-oncology-interventionalist-part-2 (accessed on 27 July 2022).
- Valgimigli, M.; Patialiakas, A.; Thury, A.; McFadden, E.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I.; Tondi, S.; Roffi, M.; et al. Zotarolimus-Eluting Versus Bare-Metal Stents in Uncertain Drug-Eluting Stent Candidates. J. Am. Coll. Cardiol. 2015, 65, 805–815. [Google Scholar] [CrossRef]
- Liang, J.J.; Sio, T.T.; Slusser, J.P.; Lennon, R.J.; Miller, R.C.; Sandhu, G.; Prasad, A. Outcomes After Percutaneous Coronary Intervention With Stents in Patients Treated With Thoracic External Beam Radiation for Cancer. JACC Cardiovasc. Interv. 2014, 7, 1412–1420. [Google Scholar] [CrossRef]
- Hernandez, J.M.D.L.T.; Hernandez, F.H.; Alfonso, F.; Rumoroso, J.R.; Lopez-Palop, R.; Sadaba, M.; Carrillo, P.; Rondan, J.; Lozano, I.; Nodar, J.M.R.; et al. Prospective Application of Pre-Defined Intravascular Ultrasound Criteria for Assessment of Intermediate Left Main Coronary Artery Lesions: Results From the Multicenter LITRO Study. J. Am. Coll. Cardiol. 2011, 58, 351–358. [Google Scholar] [CrossRef]
- Richards, G.; Johnson, T. A Vision Of Percutaneous Coronary Revascularisation In 2021: How to take advantage of intra-coronary imaging to perform more effective PCI. JRSM Cardiovasc. Dis. 2021, 10, 20480040211049976. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef]
- Johnson, T.W.; Räber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Intervent. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef]
- Lotfi, A.; Jeremias, A.; Fearon, W.F.; Feldman, M.D.; Mehran, R.; Messenger, J.C.; Grines, C.L.; Dean, L.S.; Kern, M.J.; Klein, L.W. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography. Catheter. Cardiovasc. Interv. 2014, 83, 509–518. [Google Scholar] [CrossRef]
- Iliescu, C.A.; Cilingiroglu, M.; Giza, D.E.; Rosales, O.; Lebeau, J.; Guerrero-Mantilla, I.; Lopez-Mattei, J.; Song, J.; Silva, G.; Loyalka, P.; et al. “Bringing on the light” in a complex clinical scenario: Optical coherence tomography–guided discontinuation of antiplatelet therapy in cancer patients with coronary artery disease (PROTECT-OCT registry). Am. Heart J. 2017, 194, 83–91. [Google Scholar] [CrossRef]
| Study (Year) | Population | Outcomes following PCI |
|---|---|---|
| Thomason et al. (2022) [34] | Case-control study of 2016 US NIS using machine learning to examine 30,195,722 hospitalized patients, of whom 1.27% had gynaecological cancer of whom 0.02% underwent PCI. | Among gynecological cancer patients, mortality was significantly reduced by undergoing PCI (OR 0.58, 95% CI 0.39–0.85; p = 0.006). PCI reduced mortality but not significantly for metastatic patients (OR 0.74, 95% CI 0.32–1.71; p = 0.481) |
| Mohamed et al. (2021) [35] | US NIS population hospitalised with STEMI from 2004 to 2015 with estimation of treatment effect of PPCI amongst cancer patients. (cancer N = 38,932) | Patients with cancer received PPCI less frequently (54–70% vs. 82% in no cancer). PPCI associated with lower adjusted probabilities of MACCE and all-cause mortality in the cancer groups compared with the no cancer group |
| Kwok et al. (2021) [25] | US Nationwide Readmission Database from 2010 to 2014 (cancer N = 51,289) | 90-day readmission after AMI following PCI was higher in patients with active cancer (7–12% vs. 5.6% in no cancer) 90-day readmission for bleeding post PCI was higher in cancer patients (0.6–4.2% vs. 0.6% in patients with no cancer. |
| Potts et al. (2020) [29] | US NIS sample of leukemia patients undergoing PCI between 2004 and 2014, CLL accounting for 75% of the cohort. (cancer N = 15,789) | After multivariable adjustment, leukemia patients had significantly increased odds of in-hospital mortality (OR 1.41, 95% CI 1.11–1.79) and bleeding (OR 1.87, 95% CI 1.56–2.09). |
| Velders et al. (2020) [36] | National Swedish quality registries- all patients admitted with AMI between 2001 and 2014 (cancer N = 16,237). | During a median follow up of 4.3 years, cancer was associated with all-cause mortality (HR 1.44, 95% 1.40–1.47), recurrent MI (HR 1.08, 95% CI 1.04–1.12), heart failure (HR 1.10, 95% CI 1.06–1.13) and major bleeding (HR 1.45, 95% CI 1.34–1.57) |
| Potts et al. (2019) [24] | US NIS population undergoing PCI between 2004 and 2014, having current and previous cancer rates of 1.8% and 5.8% respectively. | Patients with current lung cancer had greater in-hospital mortality (OR 2.81, 95% CI 2.37–3.34) and any in-hospital complication (OR 1.21, 95% CI 1.10–1.36), while current colon cancer was associated with any complication and bleeding. |
| Borovac et al. (2019) [37] | US NIS sample patients undergoing PCI between 2004 and 2014, 0.25% of which had a diagnosis of lymphoma (N = 18,052). | Lymphoma was associated with increased odds of in-hospital mortality (OR 1.39, 95% CI 1.25–1.54), stroke or transient ischemic attack (OR 1.75, 95% CI 1.61–1.90) and any in-hospital complication (OR 1.31, 95% CI 1.25–1.27), following PCI. |
| Tabata et al. (2018) [33] | Kumamoto University malignancy and Atherosclerosis Study (KUMA) study looking at cancer and no-cancer patients undergoing PCI | The malignant group had had a significantly higher probability of TLR than the non-malignant group (p = 0.002), with proportional hazards analyses identifying malignancy as an independent predictor of TLR (HR 2.28, 95% CI 1.3–4.0; p = 0.004) |
| Landes et al. (2017) [38] | Retrospective analysis of 12785 patients undergoing PCI (1005 cancer patients), Rabin institution, Israel. | Cancer patients had increased all-cause mortality (HR 1.46, 95% CI 1.24–1.72), death or non-fatal MI (HR 1.40, 95% CI 1.20–1.64). |
| Guddati et al. (2016) [39] | US NIS looking at patients with metastatic cancer (N = 49,515) and ACS between 2000 and 2009. | The adjusted odds of receiving PCI in patients with metastatic disease increased by 1,14 every year in the last decade, with the beneficial effect of PCI on in-hospital mortality having been noted to decline in NSTEMI such that by 2009, there was no significant difference between patients who received PCI and those who did not. |
| Wang et al. (2016) [40] | Retrospective cohort in Mayo clinic PCI registry of 2346 patients admitted with STEMI between 200 and 2010 (cancer N = 261) | At 5 years, patients with cancer had similar cardiac mortality (4.2% vs. 5.8%; HR=1.27; 95% CI, 0.77–2.10; p = 0.35), despite being seen to experience more heart failure hospitalizations (15% vs. 10%; HR=1.72; 95% CI, 1.18–2.50; p = 0.01). Cancer was associated with higher in-hospital non-cardiac mortality (1.9% vs. 0.4%, p = 0.03) |
| Hess et al. (2015) [11] | Stented patients at Duke (1996–2010) using the Duke Information Systems for Cardiovascular care and the Duke Tumor Registry.(total patients 15,008; cancer N = 496) | Post-PCI, cancer was associated with cardiovascular mortality (adjusted HR= 1.51; 95% CI 1.11 to 2.03). Composite cardiac death, MI or revascularisation was similar between pre-PCI cancer and no-cancer patient groups. |
| Study | Design | Population | Stent Type | Follow-up (Months) | Primary Outcome | Result |
|---|---|---|---|---|---|---|
| ONYX ONE (2020) [56] | Single blinded RCT | 1996 | Resolute Onyx vs. Biofreedom polymer-free DCS | 12 | Cardiac death/MI/ST | Primary outcome for Resolute Onyx vs. Biofreedom 17.1% vs. 16.9%, p = 0.011 for non-inferiority |
| STOP-DAPT-2 (2019) [57] | Open-label RCT | 3045 | Xience DES | 12 | Composite death, ST and bleeding in 1-month vs. 12-month DAPT | Composite end-point of 1-month vs. 12-month DAPT 2.4% vs. 3.7%, p < 0.001 for non-inferiority |
| GLOBAL-LEADERS (2018) [53] | Double-blinded RCT | 15 968 | Biolimus eluting stent | 24 | All-cause mortality+ MI | Primary outcome for 1-month DAPT followed by 23months ticagrelor vs. 12-month DAPT 3.8% vs. 4.4%, p = 0.073 |
| LEADERS FREE (2015) [55] | Double-blinded RCT | 2466 | Biofreedom DCS vs. BMS | 13 | Composite cardiac death/MI/ST for Biofreedom vs. BMS | Primary outcome for Biofreedom vs. BMS 9.4% vs. 12.9%, p < 0.001 for non-inferiority |
| ZEUS (2015) [58] | Single-blinded RCT | 1606 | ZES vs. BMS | 12 | MACE at 12months (all-cause mortality, MI, TVR) | ZES vs. BMS 17.5% vs. 22.1%, p = 0.011 |
| MASTER DAPT [52] | RCT | 4579 HBR patients | Bioresorbable polymer-sirolimus eluting stent | 12 | Composite all-cause death, MI, stroke or major bleeding | Net adverse clinical events 7.5% in abbreviated vs. 7.7% in standard group |
| XIENCE 90/28 [59] | RCT | 3652 HBR patients | 1-month (XIENCE 28 USA/Global) or 3-months (XIENCE 90) vs. standard DAPT for 12months | 12 | Composite all-cause death/MI | 1-month DAPT associated with similar ischemic outcomes (7.3% vs. 7.5%) and lower BARC2-5 bleeding risks (7.6% vs. 10.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doolub, G.; Mamas, M.A. Percutaneous Coronary Angioplasty in Patients with Cancer: Clinical Challenges and Management Strategies. J. Pers. Med. 2022, 12, 1372. https://doi.org/10.3390/jpm12091372
Doolub G, Mamas MA. Percutaneous Coronary Angioplasty in Patients with Cancer: Clinical Challenges and Management Strategies. Journal of Personalized Medicine. 2022; 12(9):1372. https://doi.org/10.3390/jpm12091372
Chicago/Turabian StyleDoolub, Gemina, and Mamas A. Mamas. 2022. "Percutaneous Coronary Angioplasty in Patients with Cancer: Clinical Challenges and Management Strategies" Journal of Personalized Medicine 12, no. 9: 1372. https://doi.org/10.3390/jpm12091372