Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
2.1. Study Materials
2.2. Clinical Measures and Demographics
2.3. Machine Learning Algorithms
2.4. Statistical Analyses and Machine Learning Framework
2.5. Ethical Considerations
3. Results
3.1. Sample Characteristics
3.2. Performance of Classification Algorithms
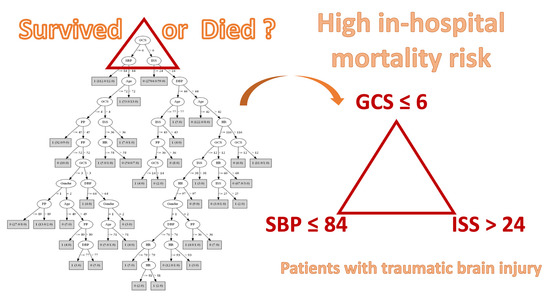
3.3. Algorithms for Predicting the In-Hospital Mortality of TBI Patients
4. Discussion
4.1. Summary of the Results
4.2. The Machine Learning Algorithms
4.3. Implications of the Findings
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, T.S.; Jing, R.; McFaull, S.R.; Cusimano, M.D. Health & economic burden of traumatic brain injury in the emergency department. Can. J. Neurol. Sci. 2016, 43, 238–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.; Maas, A. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public Health 2016, 1, e76–e83. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths-United States, 2007 and 2013. Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Korley, F.K.; Kelen, G.D.; Jones, C.M.; Diaz-Arrastia, R. Emergency department evaluation of traumatic brain injury in the United States, 2009–2010. J. Head Trauma Rehabil. 2016, 31, 379–387. [Google Scholar] [CrossRef] [Green Version]
- van Dijck, J.T.J.M.; Bartels, R.H.M.A.; Lavrijsen, J.C.M.; Ribbers, G.M.; Kompanje, E.J.O.; Peul, W.C. The patient with severe traumatic brain injury: Clinical decision-making: The first 60 min and beyond. Curr. Opin. Crit. Care 2019, 25, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Staton, C.A.; Msilanga, D.; Kiwango, G.; Vissoci, J.R.; de Andrade, L.; Lester, R.; Hocker, M.; Gerardo, C.J.; Mvungi, M. A prospective registry evaluating the epidemiology and clinical care of traumatic brain injury patients presenting to a regional referral hospital in Moshi, Tanzania: Challenges and the way forward. Int. J. Inj. Contr. Saf. Promot. 2017, 24, 69–77. [Google Scholar] [CrossRef]
- Osler, T.; Cook, A.; Glance, L.G.; Lecky, F.; Bouamra, O.; Garrett, M.; Buzas, J.S.; Hosmer, D.W. The differential mortality of Glasgow Coma Score in patients with and without head injury. Injury 2016, 47, 1879–1885. [Google Scholar] [CrossRef]
- Amorim, R.L.; Oliveira, L.M.; Malbouisson, L.M.; Nagumo, M.M.; Simoes, M.; Miranda, L.; Bor-Seng-Shu, E.; Beer-Furlan, A.; De Andrade, A.F.; Rubiano, A.M.; et al. Prediction of early TBI mortality using a machine learning approach in a LMIC population. Front. Neurol. 2020, 10, 1366. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.F.; Tsai, Y.C.; Rau, C.S.; Hsu, S.Y.; Chien, P.C.; Hsieh, H.Y.; Hsieh, C.H. Systolic blood pressure lower than the heart rate indicates a poor outcome in patients with severe isolated traumatic brain injury: A cross-sectional study. Int. J. Surg. 2019, 61, 48–52. [Google Scholar] [CrossRef]
- Najafi, Z.; Zakeri, H.; Mirhaghi, A. The accuracy of acuity scoring tools to predict 24-h mortality in traumatic brain injury patients: A guide to triage criteria. Int. Emerg. Nurs. 2018, 36, 27–33. [Google Scholar] [CrossRef]
- Huang, Y.T.; Huang, Y.H.; Hsieh, C.H.; Li, C.J.; Chiu, I.M. Comparison of injury severity score, Glasgow coma scale, and revised trauma score in predicting the mortality and prolonged ICU stay of traumatic young children: A cross-sectional retrospective study. Emerg. Med. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Majdan, M.; Brazinova, A.; Rusnak, M.; Leitgeb, J. Outcome prediction after traumatic brain injury: Comparison of the performance of routinely used severity scores and multivariable prognostic models. J. Neurosci. Rural Pract. 2017, 8, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Trickey, A.W. Classification of traumatic brain injury severity using retrospective data. J. Nurs. Educ. Pract. 2017, 7, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Champion, H.R. Trauma scoring. Scand. J. Surg. 2002, 91, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Ichikawa, R.; Misawa, J.; Shibuya, A.; Hishiki, T.; Maeda, T.; Yoshino, A.; Kondo, Y. External validation of the TRISS, CRASH, and IMPACT prognostic models in severe traumatic brain injury in Japan. PLoS ONE 2019, 14, e0221791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuenzler, M.; Braun, C.T.; Maeder, M.B. Mortality and outcome of severe traumatic brain injury in a Swiss level one trauma center. Emerg. Med. Open Access 2015, 5, 1000226. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.H.; Seo, B.R.; Jang, J.W.; Lee, J.K.; Moon, H.S. Evaluation of probability of survival using trauma and injury severity score method in severe neurotrauma patients. J. Korean Neurosurg. Soc. 2013, 54, 42–46. [Google Scholar] [CrossRef]
- Bhandarkar, P.; Munivenkatappa, A.; Roy, N.; Kumar, V.; Samudrala, V.D.; Kamble, J.; Agrawal, A. On-admission blood pressure and pulse rate in trauma patients and their correlation with mortality: Cushing’s phenomenon revisited. Int. J. Crit. Illn. Inj. Sci. 2017, 7, 14–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahraman, S.; Dutton, R.P.; Hu, P.; Stansbury, L.; Xiao, Y.; Stein, D.M.; Scalea, T.M. Heart rate and pulse pressure variability are associated with intractable intracranial hypertension after severe traumatic brain injury. J. Neurosurg. Anesthesiol. 2010, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.; Chaikittisilpa, N.; Kiatchai, T.; Vavilala, M. Hypertension after severe traumatic brain injury. J. Neurosurg. Anesthesiol. 2017, 29, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Sherrill, D.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Viscusi, C.; Mullins, T.; et al. Mortality and prehospital blood pressure in patients with major traumatic brain injury: Implications for the hypotension threshold. JAMA Surg. 2017, 152, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Adelson, P.D.; Keim, S.M.; Viscusi, C.; et al. Association of out-of-hospital hypotension depth and duration with traumatic brain injury mortality. Ann. Emerg. Med. 2017, 70, 522–530.e1. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.N.; Millham, F.H.; Chang, Y.; Fikry, K.; Alam, H.B.; King, D.R.; Velmahos, G.C.; De Moya, M.A. Presenting blood pressure in traumatic brain injury: A bimodal distribution of death. J. Trauma Inj. Infect. Crit. Care 2011, 71, 1179–1184. [Google Scholar] [CrossRef]
- Berry, C.; Ley, E.J.; Bukur, M.; Margulies, D.R.; Mirocha, J.; Salim, A. Redefining hypotension in traumatic brain injury. Injury 2012, 43, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.V.; Carmona, M.J.C.; Malbouisson, L.M.; Rizoli, S.; Rocha-Filho, J.A.; Cardoso, R.G.; Auler-Junior, J.O. Independent early predictors of mortality in polytrauma patients: A prospective, observational, longitudinal study. Clinics 2017, 72, 461–468. [Google Scholar] [CrossRef]
- Chang, J.J.; Khorchid, Y.; Dillard, K.; Kerro, A.; Burgess, L.G.; Cherkassky, G.; Goyal, N.; Chapple, K.; Alexandrov, A.W.; Buechner, D.; et al. Elevated pulse pressure levels are associated with increased in-hospital mortality in acute spontaneous intracerebral hemorrhage. Am. J. Hypertens. 2017, 30, 719–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenell, S.; Nyholm, L.; Lewén, A.; Enblad, P. Clinical outcome and prognostic factors in elderly traumatic brain injury patients receiving neurointensive care. Acta Neurochir. 2019, 161, 1243–1254. [Google Scholar] [CrossRef] [Green Version]
- Areas, F.Z.; Schwarzbold, M.L.; Diaz, A.P.; Rodrigues, I.K.; Sousa, D.S.; Ferreira, C.L.; Quevedo, J.; Lin, K.; Kupek, E.; Ritter, C.; et al. Predictors of hospital mortality and the related burden of disease in severe traumatic brain injury: A prospective multicentric study in Brazil. Front. Neurol. 2019, 10, 432. [Google Scholar] [CrossRef]
- Haring, R.S.; Narang, K.; Canner, J.K.; Asemota, A.O.; George, B.P.; Selvarajah, S.; Haider, A.H.; Schneider, E.B. Traumatic brain injury in the elderly: Morbidity and mortality trends and risk factors. J. Surg. Res. 2015, 195, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Siddiq, F.; Qureshi, A.I. Prehospital neurologic deterioration is independent predictor of outcome in traumatic brain injury: Analysis from National Trauma Data Bank. Am. J. Emerg. Med. 2013, 31, 1215–1219. [Google Scholar] [CrossRef]
- Pruitt, P.; Penn, J.; Peak, D.; Borczuk, P. Identifying patients with mild traumatic intracranial hemorrhage at low risk of decompensation who are safe for ED observation. Am. J. Emerg. Med. 2017, 35, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA workbench. In Data Mining: Practical Machine Learning Tools and Techniques, 4th ed.; Morgan Kaufmann: Burlington, MA, USA, 2016; pp. 553–571. [Google Scholar] [CrossRef]
- Tezza, F.; Lorenzoni, G.; Azzolina, D.; Barbar, S.; Leone, L.A.C.; Gregori, D. Predicting in-hospital mortality of patients with COVID-19 using machine learning techniques. J. Pers. Med. 2021, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Maimon, O.; Rokach, L. Decision trees. In Data Mining and Knowledge Discovery Handbook; Rokach, L., Maimon, O., Eds.; Springer: Boston, MA, USA, 2005; pp. 165–192. [Google Scholar]
- Mesarić, J.; Šebalj, D. Decision trees for predicting the academic success of students. Croat. Oper. Res. Rev. 2016, 7, 367–388. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Thakare, S.; Jaiswal, A. Survey on classification techniques for data mining. Int. J. Comput. Appl. 2015, 132, 13–16. [Google Scholar] [CrossRef]
- Schiaffino, S.; Codari, M.; Cozzi, A.; Albano, D.; Alì, M.; Arioli, R.; Avola, E.; Bnà, C.; Cariati, M.; Carriero, S.; et al. Machine learning to predict in-hospital mortality in covid-19 patients using computed tomography-derived pulmonary and vascular features. J. Pers. Med. 2021, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Liu, H.E.; Goopy, S.; Chen, L.C.; Chen, H.J.; Han, C.Y. Using the five-level Taiwan triage and acuity scale computerized system: Factors in decision making by emergency department triage nurses. Clin. Nurs. Res. 2017, 26, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Luostarinen, T.; Pursiainen, E.; Posti, J.P.; Takala, R.S.K.; Bendel, S.; Konttila, T.; Korja, M. Machine learning-based dynamic mortality prediction after traumatic brain injury. Sci. Rep. 2019, 9, 17672. [Google Scholar] [CrossRef]
- Jeni, L.A.; Cohn, J.F.; De La Torre, F. Facing Imbalanced Data—Recommendations for the Use of Performance Metrics. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Geneva, Switzerland, 2–5 September 2013; IEEE: Geneva, Switzerland, 2013; pp. 245–251. [Google Scholar] [CrossRef] [Green Version]
- Halligan, S.; Altman, D.G.; Mallett, S. Disadvantages of using the area under the receiver operating characteristic curve to assess imaging tests: A discussion and proposal for an alternative approach. Eur. Radiol. 2015, 25, 932–939. [Google Scholar] [CrossRef] [Green Version]


| Variables | All (N = 3331) | Lived (n = 3003) | Died (n = 328) | p-Value c | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Gender (n, %) | 0.622 | ||||||
| Male a | 2221 | 66.68 | 1998 | 90.00 | 223 | 10.00 | |
| Female a | 1110 | 33.32 | 1005 | 90.54 | 105 | 9.46 | |
| Age | 51.14 | 29.17 | 50.42 | 29.66 | 57.79 | 23.24 | <0.001 |
| SBP | 137.54 | 42.03 | 140.64 | 33.42 | 109.15 | 82.74 | <0.001 |
| DBP | 78.70 | 23.93 | 80.79 | 19.83 | 59.52 | 42.60 | <0.001 |
| PP | 58.84 | 27.53 | 59.85 | 22.80 | 49.63 | 53.38 | <0.001 |
| HR | 85.00 | 24.09 | 86.27 | 19.85 | 73.37 | 46.30 | <0.001 |
| ISS | 16.09 | 8.64 | 14.95 | 7.38 | 26.53 | 11.78 | <0.001 |
| GCS | 12.97 | 3.70 | 13.69 | 2.78 | 6.36 | 4.44 | <0.001 |
| Model | Accuracy | Precision | Recall | F1 | AUC | Success Rate, Class “Lived” | Success Rate, Class “Died” | Average Success Rate |
|---|---|---|---|---|---|---|---|---|
| J48 | 93.2 | 92.7 | 93.2 | 92.9 | 82.0 | 97.1 | 57.3 | 77.2 |
| Random Forest | 93.3 | 92.7 | 93.3 | 92.9 | 92.1 | 97.8 | 52.4 | 75.1 |
| Random Tree | 91.0 | 90.8 | 91.0 | 90.9 | 73.5 | 95.2 | 51.8 | 73.5 |
| REP Tree | 92.0 | 91.1 | 92.0 | 91.4 | 84.6 | 96.9 | 46.3 | 71.6 |
| KNN | 91.0 | 90.5 | 91.0 | 90.7 | 71.6 | 95.7 | 48.2 | 72.0 |
| SVM | 93.2 | 92.5 | 93.2 | 92.3 | 71.0 | 98.7 | 43.3 | 71.0 |
| NB | 91.9 | 91.7 | 91.9 | 91.8 | 91.7 | 95.7 | 56.4 | 76.1 |
| Outcomes | Predicted Outcome | ||
|---|---|---|---|
| Alive | Died | ||
| Actual outcome | Alive | 2915 | 88 |
| Died | 140 | 188 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-D.; Chao, E.; Chen, S.-J.; Hueng, D.-Y.; Lan, H.-Y.; Chiang, H.-H. Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury. J. Pers. Med. 2021, 11, 1144. https://doi.org/10.3390/jpm11111144
Hsu S-D, Chao E, Chen S-J, Hueng D-Y, Lan H-Y, Chiang H-H. Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury. Journal of Personalized Medicine. 2021; 11(11):1144. https://doi.org/10.3390/jpm11111144
Chicago/Turabian StyleHsu, Sheng-Der, En Chao, Sy-Jou Chen, Dueng-Yuan Hueng, Hsiang-Yun Lan, and Hui-Hsun Chiang. 2021. "Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury" Journal of Personalized Medicine 11, no. 11: 1144. https://doi.org/10.3390/jpm11111144