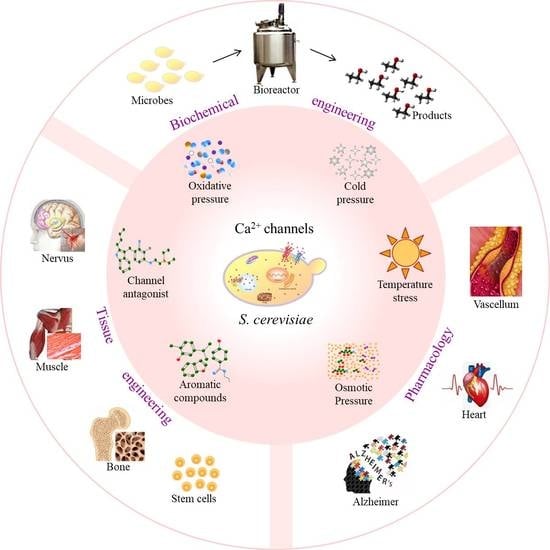
Calcium Ion Channels in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Types and Characteristics of Ca2+ Channels in S. cerevisiae
2.1. VGCC
2.2. SACC
2.3. TRPY1
3. Gating Mechanisms of Ca2+ Channels
3.1. Voltage-Dependent Gating
3.2. Stretch-Activated Gating
3.3. Transient Receptor Gating
3.4. Factors Affecting Ca2+ Channel Gating States
4. Applications of Ca2+ Channels in S. cerevisiae
4.1. Pharmacology
4.2. Tissue Engineering
4.3. Biochemical Engineering
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signaling: Dynamics, homeostasis and remodeling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Sze, H.; Liang, F.; Hwang, I.; Curran, A.C.; Harper, J.F. Diversity and regulation of plant Ca2+ pumps: Insights from expression in yeast. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2000, 51, 433–462. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.M.; Pearce, D.A. Channeling studies in yeast as a model for channelopathies? Neuromol. Med. 2006, 8, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y. Enhanced ethanol production of Saccharomyces cerevisiae induced by cold plasma at atmospheric air. In Fuel Ethanol Production from Sugarcane, 1st ed.; Basso, T.P., Basso, L.C., Eds.; IntechOpen: London, UK, 2019; pp. 155–175. [Google Scholar]
- Palmer, C.P.; Zhou, X.L.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef]
- Tada, T.; Ohmori, M.; Iida, H. Molecular dissection of the hydrophobic segments H3 and H4 of the yeast Ca2+ channel component Mid1. J. Biol. Chem. 2003, 278, 9647–9654. [Google Scholar] [CrossRef]
- Teng, J.; Goto, R.; Iida, K.; Kojima, I.; Iida, H. Ion-channel blocker sensitivity of voltage-gated calcium-channel homologue Cch1 in Saccharomyces cerevisiae. Microbiology 2008, 154, 3775–3781. [Google Scholar] [CrossRef]
- Teng, J.; Iida, K.; Imai, A.; Nakano, M.; Tada, T.; Iida, H. Hyperactive and hypoactive mutations in Cch1, a yeast homologue of the voltage-gated calcium-channel pore-forming subunit. Microbiology 2013, 159, 970–979. [Google Scholar] [CrossRef]
- Fischer, M.; Schnell, N.; Chattaway, J.; Davies, P.; Dixon, G.; Sanders, D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997, 419, 259–262. [Google Scholar] [CrossRef]
- Chandel, A.; Bachhawat, A.K. Redox regulation of the yeast voltage-gated Ca2+ channel homolog Cch1p by glutathionylation of specific cysteine residues. J. Cell Sci. 2017, 130, 2317–2328. [Google Scholar] [CrossRef]
- Hong, M.P.; Vu, K.; Bautos, J.; Gelli, A. Cch1 restores intracellular Ca2+ in fungal cells during endoplasmic reticulum stress. J. Biol. Chem. 2010, 285, 10951–10958. [Google Scholar] [CrossRef]
- Cibulsky, S.M.; Sather, W.A. The EEEE locus is the sole high-affinity Ca2+ binding structure in the pore of a voltage-gated Ca2+ channel: Block by Ca2+ entering from the intracellular pore entrance. J. Gen. Physiol. 2000, 116, 349–362. [Google Scholar] [CrossRef]
- Prole, D.L.; Taylor, C.W. Identification and analysis of cation channel homologues in human pathogenic fungi. PLoS ONE 2012, 7, e42404. [Google Scholar] [CrossRef]
- Maruoka, T.; Nagasoe, Y.; Inoue, S.; Mori, Y.; Goto, J.; Ikeda, M.; Iida, H. Essential hydrophilic carboxyl-terminal regions including cysteine residues of the yeast stretch-activated calcium-permeable channel Mid1. J. Biol. Chem. 2002, 277, 11645–11652. [Google Scholar] [CrossRef]
- Tada, T.; Ohmori, M.; Iida, H. Phe356 in the yeast Ca2+ channel component Mid1 is a key residue for viability after exposure to α-factor. Biochem. Biophys. Res. Commun. 2004, 313, 752–757. [Google Scholar] [CrossRef]
- Iida, K.; Teng, J.; Cho, T.; Yoshikawa-Kimura, S.; Iida, H. Post-translational processing and membrane translocation of the yeast regulatory Mid1 subunit of the Cch1/VGCC/NALCN cation channel family. J. Biol. Chem. 2017, 292, 20570–20582. [Google Scholar] [CrossRef]
- Yoshimura, H.; Tada, T.; Iida, H. Subcellular localization and oligomeric structure of the yeast putative stretch-activated Ca2+ channel component Mid1. Exp. Cell. Res. 2004, 293, 185–195. [Google Scholar] [CrossRef]
- Christensen, A.P.; Corey, D.P. TRP channels in mechanosensation: Direct or indirect activation? Nat. Rev. Neurosci. 2007, 8, 510–521. [Google Scholar] [CrossRef]
- Chang, Y.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Properties of the intracellular transient receptor potential (TRP) channel in Yeast, Yvc1. FEBS Lett. 2010, 584, 2028–2032. [Google Scholar] [CrossRef]
- Su, Z.; Zhou, X.; Loukin, S.H.; Saimi, Y.; Kung, C. Mechanical force and cytoplasmic Ca2+ activate yeast TRPY1 in parallel. J. Membr. Biol. 2009, 227, 141–150. [Google Scholar] [CrossRef]
- Chandel, A.; Das, K.K.; Bachhawat, A.K. Glutathione depletion activates the yeast vacuolar transient receptor potential channel, Yvc1p, by reversible glutathionylation of specific cysteines. Mol. Biol. Cell. 2016, 27, 3913–3925. [Google Scholar] [CrossRef]
- Ahmed, T.; Nisler, C.R.; Fluck, E.C., 3rd; Walujkar, S.; Sotomayor, M.; Moiseenkova-Bell, V.Y. Structure of the ancient TRPY1 channel from Saccharomyces cerevisiae reveals mechanisms of modulation by lipids and calcium. Structure 2022, 30, 139–155.e5. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Tada, T.; Iida, H. Molecular cloning in yeast by in vivo homologous recombinant of the yeast putative α1 subunit of the voltage-gated calcium channel. FEBS Lett. 2004, 576, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Qian, X.; Lu, S.; Dong, M.; Zhou, Q.; Yan, N. Structure of the voltage-gated calcium channel Cav1.1 at 3.6 Å resolution. Nature 2016, 537, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Yan, C.; Lu, S.; Dong, M.; Yan, N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science 2015, 350, 1492. [Google Scholar] [CrossRef] [PubMed]
- Beyl, S.; Depil, K.; Hohaus, A.; Stary-Weinzinger, A.; Linder, T.; Timin, E.; Hering, S. Neutralisation of a single voltage sensor affects gating determinants in all four pore-forming S6 segments of Cav1.2: A cooperative gating model. Pflügers Arch. Eur. J. Physiol. 2012, 464, 391–401. [Google Scholar] [CrossRef]
- Xie, C.; Zhen, X.; Yang, J. Localization of the activation gate of a voltage-gated Ca2+ channel. J. Gen. Physiol. 2005, 126, 205–212. [Google Scholar] [CrossRef]
- Iida, K.; Teng, J.; Tada, T.; Saka, A.; Tamai, M.; Izumi-Nakaseko, H.; Adachi-Akahane, S.; Iida, H. Essential, completely conserved glycine residue in the domain III S2-S3 linker of voltage-gated calcium channel alpha1 subunits in yeast and mammals. J. Biol. Chem. 2007, 282, 25659–25667. [Google Scholar] [CrossRef]
- Hayashi, T.; Oishi, K.; Kimura, M.; Iida, K.; Iida, H. Highly conserved extracellular residues mediate interactions between pore-forming and regulatory subunits of the yeast Ca2+ channel related to the animal VGCC/NALCN family. J. Biol. Chem. 2020, 295, 13008–13022. [Google Scholar] [CrossRef]
- Tanifuji, M.; Sato, M.; Wada, Y.; Anraku, Y.; Kasai, M. Gating behaviors of a voltage-dependent and Ca2+-activated cation channel of yeast vacuolar membrane incorporated into planar lipid bilayer. J. Membr. Biol. 1988, 106, 47–55. [Google Scholar] [CrossRef]
- Zhou, X.L.; Batiza, A.F.; Loukin, S.H.; Palmer, C.P.; Kung, C.; Saimi, Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. USA 2003, 100, 7105–7110. [Google Scholar] [CrossRef]
- Hamill, O.P.; Martinac, B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001, 81, 685–740. [Google Scholar] [CrossRef]
- Viladevall, L.; Serrano, R.; Ruiz, A.; Domenech, G.; Giraldo, J.; Barcelo, A.; Arino, J. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 43614–43624. [Google Scholar] [CrossRef]
- Liu, M.; Du, P.; Heinrich, G.; Cox, G.M.; Gelli, A. Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot. Cell 2006, 5, 1788–1796. [Google Scholar] [CrossRef]
- Peiter, E.; Fischer, M.; Sidaway, K.; Roberts, S.K.; Sanders, D. The Saccharomyces cerevisiae Ca2+ channel Cch1pMid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett. 2005, 579, 5697–5703. [Google Scholar] [CrossRef]
- Maresova, L.; Muend, S.; Zhang, Y.Q.; Sychrova, H.; Rao, R. Membrane hyperpolarization drives cation influx and fungicidal activit of amiodarone. J. Biol. Chem. 2009, 284, 2795–2802. [Google Scholar] [CrossRef]
- Muend, S.; Rao, R. Fungicidal activity of amiodarone is tightly coupled to calcium influx. FEMS Yeast Res. 2008, 8, 425–431. [Google Scholar] [CrossRef]
- Pereira, R.S. The use of calcium blockers to study biochemical behavior of Saccharomyces cerevisiae cells. Mol. Cell. Biochem. 2001, 228, 1–7. [Google Scholar] [CrossRef]
- Haynes, W.J.; Zhou, X.L.; Su, Z.W.; Loukin, S.H.; Saimi, Y.; Kung, C. Indole and other aromatic compounds activate the yeast TRPY1 channel. FEBS Lett. 2008, 582, 1514–1518. [Google Scholar] [CrossRef]
- Lee, J.H.; Wendisch, V.F. Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass. J. Biotechnol. 2017, 257, 211–221. [Google Scholar] [CrossRef]
- Monk, B.C.; Goffeau, A. Outwitting multidrug resistance to antifungals. Science 2008, 321, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K.; McAinsh, M.; Cantopher, H.; Sandison, S. Calcium dependence of eugenol tolerance and toxicity in Saccharomyces cerevisiae. PLoS ONE 2014, 9, e102712. [Google Scholar] [CrossRef] [PubMed]
- Bogeski, I.; Kummerow, C.; Al-Ansary, D.; Schwarz, E.C.; Koehler, R.; Kozai, D.; Takahashi, N.; Peinelt, C.; Griesemer, D.; Bozem, M.; et al. Differential redox regulation of ORAI ion channels: A mechanism to tune cellular calcium signaling. Sci. Signal. 2010, 3, ra24. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Yuan, X.; Wang, R.J. Interaction of air cold plasma with Saccharomyces cerevisiae in the multi-scale microenvironment for improved ethanol yield. Bioresour. Technol. 2021, 323, 124621. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, S.; Mori, Y.; Yabe, I.; Uozumi, N. In vitro and in vivo characterization of modulation of the vacuolar cation channel TRPY1 from Saccharomyces cerevisiae. FEBS J. 2018, 285, 1146–1161. [Google Scholar] [CrossRef]
- Popa, C.V.; Dumitru, I.; Ruta, L.L.; Danet, A.F.; Farcasanu, I.C. Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J. 2010, 277, 4027–4038. [Google Scholar] [CrossRef]
- Courchesne, W.E.; Vlasek, C.; Klukovich, R.; Coffee, S. Ethanol induces calcium influx via the Cch1-Mid1 transporter in Saccharomyces cerevisiae. Arch. Microbiol. 2011, 193, 323–334. [Google Scholar] [CrossRef]
- Groppi, S.; Belotti, F.; Brandao, R.L.; Martegani, E.; Tisi, R. Glucose-induced calcium influx in budding yeast involves a novel calcium transport system and can activate calcineurin. Cell Calcium 2011, 49, 376–386. [Google Scholar] [CrossRef]
- Cui, J.; Kaandorp, J.A.; Sloot, P.M.A.; Lloyd, C.M.; Filatov, M.V. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res. 2009, 9, 1137–1147. [Google Scholar] [CrossRef]
- Imaizumi, K.; Katayama, T.; Tohyma, M. Presenilin and the UPR. Nat. Cell Biol. 2001, 3, E104. [Google Scholar] [CrossRef]
- Paschen, W.; Frandsen, A. Endoplasmic reticulum dysfunction-a common denominator for cell injury in acute and degenerative diseases of the brain? J. Neurochem. 2002, 79, 719–725. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Fei, D.D.; He, X.N.; Dai, J.M.; Xu, R.C.; Xu, X.Y.; Wu, J.J.; Li, B. L-type voltage-gated calcium channels in stem cells and tissue engineering. Cell Proliferat 2019, 52, e12623. [Google Scholar] [CrossRef]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Cav1-channel activity. J. Cell. Physiol. 2008, 215, 129–139. [Google Scholar] [CrossRef]
- Liu, L.; Yu, B.; Sun, W.; Liang, C.; Ying, H.; Zhou, S.; Niu, H.; Wang, Y.; Liu, D.; Chen, Y. Calcineurin signaling pathway influences Aspergillus niger biofilm formation by affecting hydrophobicity and cell wall integrity. Biotechnol. Biofuels 2020, 13, 54. [Google Scholar] [CrossRef]
- Vu, K.; Bautos, J.; Hong, M.P.; Gelli, A. The functional expression of toxic genes: Lessons learned from molecular cloning of CCH1, a high-affinity Ca2+ channel. Anal. Biochem. 2009, 393, 234–241. [Google Scholar] [CrossRef]
- Dong, X.Y. Preparation and identification of monoclonal antibodies of calcium channel membrane proteins in Saccharomyces cerevisiae. Chem. Ind. Eng. Prog. 2021, 40, 334–343. [Google Scholar]



| Affecting Factors | Reagents | Calcium Ion Channels | Effects | Gating Mechanism | Applications | References |
|---|---|---|---|---|---|---|
| Calcium ion (Ca2+) channel antagonists/blockers | Amiodarone, amlodipine, diltiazem, nicardipine, nifedipine, nimodipine, nitrendipine, verapamil | Cch1, Cch1-Mid1, or Mid1 | Blocking calcium channels or stimulating channel opening | Binding to the special sites on Ca2+ channels for blockage | Clinical treatment of hypertension, coronary heart disease, and cardiac arrhythmia | [7,37,38,39] |
| Aromatic compounds | Carvacrol, eugenol, indole, methylated, propylparaben, quinolone, thymol | Cch1 or Yvc1 | Improving cell tolerance or mediating the Ca2+ increase in the cytoplasm | Competing with the aromatic residues of the channel proteins for lipid anchors or generating force profile in the bilayer for open conformation | Antifungal drugs | [40,41,42,43] |
| Oxidative stress | Chloramine T, dithiothreitol, hydrogen peroxide, 2-mercaptoethanol, mitochondrion, N-ethylmaleimide, plasma discharge, reduced glutathione, β-phenylethylamine, tert-butylhydroperoxide (tBOOH) | Cch1, Mid1, or Yvc1 | Affecting activity, expression, open-time probability, or trafficking | Specific glutathionylation of cysteine residues in the pore forming region; oxidation of special amino acids; change in the channel conformation | Experiments on channel functions or metabolic regulation | [10,44,45,46,47] |
| Osmotic pressure | Ethanol, hexose (glucose and galactose), LiCl, NaCl, sorbitol | Cch1, Mid1, or Yvc1 | Channel opening or improving ethanol tolerance | Membrane perturbation, vacuolar shrinkage and deformation, or activation of the PKC1 pathway | Industrial microbial fermentation | [32,48,49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.-Y. Calcium Ion Channels in Saccharomyces cerevisiae. J. Fungi 2023, 9, 524. https://doi.org/10.3390/jof9050524
Dong X-Y. Calcium Ion Channels in Saccharomyces cerevisiae. Journal of Fungi. 2023; 9(5):524. https://doi.org/10.3390/jof9050524
Chicago/Turabian StyleDong, Xiao-Yu. 2023. "Calcium Ion Channels in Saccharomyces cerevisiae" Journal of Fungi 9, no. 5: 524. https://doi.org/10.3390/jof9050524