Polymeric Floating Photocatalysts Based on PE/TiO2 Composites for the Removal of Organic Pollutants in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
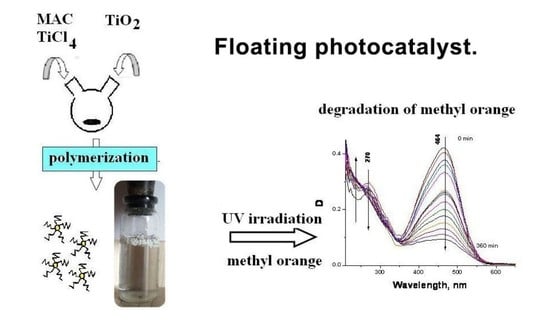
2.2. Synthesis of PE/TiO2(P25) by In Situ Ethylene Polymerization
2.3. Characterization of Samples
2.3.1. TG Analysis of Composites
2.3.2. Scanning Electron Spectroscopy (SEM)
2.3.3. A DRIFTS Study

2.3.4. UV-Vis Spectroscopy
2.4. Photodegradation of MO
3. Results
3.1. Synthesis of the PE/TiO2 Material


3.2. Characterization of FPCs
3.3. PECs Performance in Photocatalytic Degradation of Methyl Orange
3.3.1. Correlation between Adsorption and Degradation of MO
3.3.2. Effect of the Polymer Content in the PE/TiO2 Composite on Catalyst Performance
3.3.3. Effect of the MO Concentration
3.3.4. Effect of the PE/TiO2 Composite Concentration
3.3.5. Re-Use of PE/TiO2 Composites
4. Conclusions
5. Concluding Remarks and Further Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasir, A.M.; Jaafar, J.; Aziz, F.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F.; Aziz, M. A review on floating nanocomposite photocatalyst: Fabrication and applications for wastewater treatment. J. Water Process Eng. 2020, 36, 101300. [Google Scholar] [CrossRef]
- Sacco, O.; Venditto, V.; Pragliola, S.; Vaiano, V. Composite Systems Based on N-Doped TiO2/Polymeric Materials for Visible-Light-Driven Pollutant Degradation: A Mini Review. Photochem 2021, 1, 330–344. [Google Scholar] [CrossRef]
- Yañez, D.; Guerreroc, S.; Lieberwirthd, I.; Ulloa, M.T.; Gomez, T.; Rabagliati, F.M.; Zapata, P.A. Photocatalytic inhibition of bacteria by TiO2 nanotubes-doped polyethylene composites. Appl. Catal. A Gen. 2015, 489, 255–261. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Jaramillo, L.Y.; Saravanan, R.; Benito, N.; Pabón, E.; Mosquera, E.; Gracia, F. Notable photocatalytic activity of TiO2-polyethylene nanocomposites for visible light degradation of organic pollutants. Express Polym. Lett. 2017, 11, 899–909. [Google Scholar] [CrossRef]
- Prasert, A.; Sontikaew, S.; Sriprapai, D.; Chuangchote, S. Polypropylene/ZnO Nanocomposites: Mechanical Properties, Photocatalytic Dye Degradation, and Antibacterial Property. Materials 2020, 13, 914. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xu, S.; He, L.; Xu, F.; Wang, Y.; Zhang, L. Photocatalytic Degradation of Polyethylene Plastic with Polypyrrole/TiO2 Nanocomposite as Photocatalyst. Polym.-Plast. Technol. Eng. 2010, 49, 400–406. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, X.; Luo, Q.; An, J.; Yue, J. Sunlight photocatalytic activity of polypyrrole–TiO2 nanocomposites prepared by ‘in situ’ method. Catal. Commun. 2008, 9, 1162–1166. [Google Scholar] [CrossRef]
- Bassaid, S.; Benhaoua, C.; Taleb, M.; Sahli, M.; Dehbi, A. Physical and Chemical Properties of Composites Based on Polythiophene and Titanium Dioxide Nanoparticles for Photocatalysis. Polym. Sci. Ser. B 2021, 63, 291–303. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Thomas, R.T.; Nair, V.; Sandhyarani, N. TiO2 nanoparticle assisted solid phase photocatalytic degradation of polythene film: A mechanistic investigation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 1–9. [Google Scholar] [CrossRef]
- Tang, Q.; Lin, J.; Wu, Z.; Wu, J.; Huang, M.; Yang, Y. Preparation and photocatalytic degradability of TiO2/polyacrylamide composite. Eur. Polym. J. 2007, 43, 2214–2220. [Google Scholar] [CrossRef]
- Ye, J.; Chao, C.; Hong, J. Preparation of a novel nano-TiO2 photocatalytic composite using insoluble wood flour as bio-carrier and dissolved components as accelerant. J. Mater. Res. Technol. 2020, 9, 11255–11262. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Zakharov, V.A.; Matsko, M.A. Study of the Bis(imino)pyridyl Complex of Fe(II)/Nafen and Titanium-Magnesium/Nafen Catalysts for Synthesis of Polyethylene/Nafen Composites by in situ Ethylene Polymerization. Polym. Sci. Ser. B 2022, 64, 393–401. [Google Scholar] [CrossRef]
- Kostyukov, A.I.; Panchenko, V.N.; Rakhmanova, M.I.; Nashivochnikov, A.A.; Matsko, M.A.; Suprun, E.A. Optical properties of composites based on polyethylene and monoclinic Y2O3:Eu3+ nanoparticles. Mater. Chem. Phys. 2021, 273, 125140. [Google Scholar] [CrossRef]
- Wojciech, M. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Cazan, C.; Enesca, A.; Andronic, L. Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers 2021, 13, 2017. [Google Scholar] [CrossRef]
- Madani, M. Structure, optical and thermal decomposition characters of LDPE graft copolymers synthesized by gamma irradiation. Curr. Appl. Phys. 2011, 11, 70–76. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Babu, B.; Mallikarjuna, K.; Venkata Reddy, C.; Park, J. Facile synthesis of Cu@TiO2 core shell nanowires for efficient photocatalysis. Mater. Lett. 2016, 176, 265–269. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Li, H. Photocatalytic Degradation of Plastic Waste: A Mini Review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lia, Z.; Chen, Y.; Shi, L.; Zhu, Y. Solid-phase photocatalytic degradation of polyethylene plastic under UV and solar light irradiation. J. Mol. Catal. A Chem. 2007, 268, 101–106. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhu, D.W.; Liao, S.J.; Ren, L.Y.; Cui, J.Z.; Zhou, W.B. Solid-phase photocatalytic degradation of polyethylene–goethite composite film under UV-light irradiation. J. Hazard. Mater. 2009, 172, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadou, I.; Paraskevopoulos, K.M.; Chrissafis, K.; Pavlidou, E.; Stamkopoulos, T.-G.; Bikiaris, D. Effect of different nanoparticles on HDPE UV stability. Polym. Degrad. Stab. 2011, 96, 151–163. [Google Scholar] [CrossRef]
- Magalhães, F.; Lago, R.M. Floating photocatalysts based on TiO2 grafted on expanded polystyrene beads for the solar degradation of dyes. Sol. Energy 2009, 83, 1521–1526. [Google Scholar] [CrossRef]
- Baiocchi, C.; Brussino, M.C.; Pramauroa, E.; Prevot, A.B.; Palmisano, L.; Marci, G. Characterization of methyl orange and its photocatalytic degradation products by HPLC/UV–VIS diode array and atmospheric pressure ionization quadrupole ion trap mass spectrometry. Int. J. Mass Spectrom. 2002, 214, 247–256. [Google Scholar] [CrossRef]
- Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photodegradation of the aminoazobenzene acid orange 52 by three advanced oxidation processes: UV/H2O2, UV/TiO2 and VIS/TiO2. J. Photochem. Photobiol. A Chem. 2000, 130, 35–47. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, Y.; Jiang, L.; Dan, Y. Visible Light Induced Photocatalytic Degradation of Methyl Orange by Polythiophene/TiO2 Composite Particles. Water Air Soil Pollut. 2010, 213, 151–159. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic degradation of methyl orange in a sparged tube reactor with TiO2-coated activated carbon composites. Catal. Commun. 2005, 6, 650–655. [Google Scholar] [CrossRef]
- Rashed, M.N.; El-Amin, A.A. Photocatalytic degradation of methyl orange in aqueous TiO2 under different solar irradiation sources. Int. J. Phys. Sci. 2007, 2, 073–081. [Google Scholar]
- Li, Y.; Zhao, H.; Yang, M. TiO2 nanoparticles supported on PMMA nanofibers for photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2017, 508, 500–507. [Google Scholar] [CrossRef]
- XuRauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Al-Mamun, R.; Hossain, K.T.; Mondal, S.; Khatun, M.A.; Islam, S.; Khan, Z.H. Synthesis, characterization, and photocatalytic performance of methyl orange in aqueous TiO2 suspension under UV and solar light irradiation. S. Afr. J. Chem. Eng. 2022, 40, 113–125. [Google Scholar] [CrossRef]
- Iqbal, A.; Saidu, U.; Adam, F.; Sreekantan, S.; Yahaya, N.; Ahmad, M.N.; Ramalingam, R.J.; Wilson, L.D. Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation photodegradation of tetracycline (TC) antibiotics. Molecules 2021, 26, 2509. [Google Scholar] [CrossRef]











| Sample | Changing Weight at Definite Temperatures, % wt. | Total Changing Weight % wt. | Content of PE, % wt. | ||||
|---|---|---|---|---|---|---|---|
| 100–160 °C | 200–290 °C | 290–400 °C | 400–500 °C | TG-TGA | TG-TGA | For yield of PE | |
| PE/TiO2 | 5.2 | 2.2 | 15.7 | 11.7 | 34.8 | 29.6 | 38.2 |
| H-PE/TiO2 | 4.9 | 2.7 | 18.3 | 13.4 | 39.3 | 34.4 | 42.8 |
| Sample | PE, wt% | MO ppm | pH0 | pHe | Changing of MO % | |
|---|---|---|---|---|---|---|
| for 1 h Adsorption | for 4 h UV-Radiation | |||||
| 1 | 17.5 | 5 | 6.7 | 5.4 | 12.1 | 55.5 |
| 2 | 42.8 | 5 | 6.9 | 5.4 | 9.2 | 33.0 |
| 3 | 55.8 | 5 | 6.9 | 5.8 | 5.5 | 22.4 |
| 4 | 85.4 | 5 | 6.7 | 6.6 | 1.3 | 0.5 |
| Sample | Concentration of MO, ppm | SBET, m2/g | pH0 | pHe | Changing of MO % | |
|---|---|---|---|---|---|---|
| for 1 h Adsorption | for 4 h UV-Radiation | |||||
| 1 | 1.7 | - | 6.7 | 6.4 | 8.4 | 16.7 |
| 2 | 5.0 | 32.6 | 6.7 | 6.4 | 4.5 | 12.5 |
| 3 1 | 5.0 | 36.3 | 6.7 | 6.4 | 4.3 | 12.8 |
| 4 2 | 5.0 | 45.2 | 6.7 | 6.6 | 4.3 | 38.9 |
| 5 | 15.0 | - | 6.9 | 6.9 | 4.0 | 5.5 |
| 6 | 20.0 | - | 6.9 | 6.9 | 4.0 | 2.0 |
| Sample | C(MO), ppm | Load of FPC, g | pH0 | pHe | Changing of MO for 4 h UV Radiation, %wt. | |
|---|---|---|---|---|---|---|
| Total | for Load of Catalyst | |||||
| 38.2PE/TiO2 | ||||||
| 1 | 5 | 0.1 | 6.7 | 6.4 | 12.5 | 12.5 |
| 2 | 5 | 0.2 | 6.7 | 6.4 | 24.0 | 12.0 |
| 3 | 5 | 0.5 | 6.7 | 4.0 | 77.7 | 15.5 |
| H-40.5PE/TiO2 | ||||||
| 4 | 5 | 0.1 | 7.2 | 6.1 | 9.0 | 9.0 |
| 5 | 5 | 0.5 | 6.7 | 5.7 | 68.8 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, V.N.; Matsko, M.A.; Selishchev, D.S.; Kozlov, D.V. Polymeric Floating Photocatalysts Based on PE/TiO2 Composites for the Removal of Organic Pollutants in Water. J. Compos. Sci. 2023, 7, 318. https://doi.org/10.3390/jcs7080318
Panchenko VN, Matsko MA, Selishchev DS, Kozlov DV. Polymeric Floating Photocatalysts Based on PE/TiO2 Composites for the Removal of Organic Pollutants in Water. Journal of Composites Science. 2023; 7(8):318. https://doi.org/10.3390/jcs7080318
Chicago/Turabian StylePanchenko, Valentina N., Mikhail A. Matsko, Dmitry S. Selishchev, and Denis V. Kozlov. 2023. "Polymeric Floating Photocatalysts Based on PE/TiO2 Composites for the Removal of Organic Pollutants in Water" Journal of Composites Science 7, no. 8: 318. https://doi.org/10.3390/jcs7080318