Severe Cholestasis in Neonates with Hemolytic Disease of the Fetus and Newborn—A Case Report
Abstract
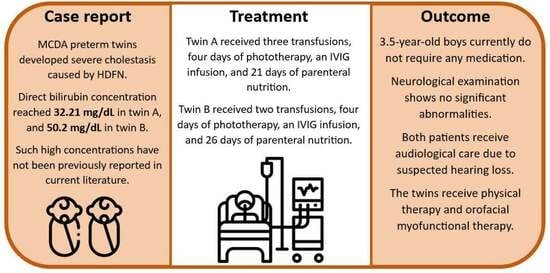
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrei, C.; Vladareanu, R. The value of reference ranges for middle cerebral artery peak systolic velocity in the management of rhesus alloimmunized pregnancies. Maedica 2012, 7, 14–19. [Google Scholar]
- Lindenburg, I.T.; van Kamp, I.L.; Oepkes, D. Intrauterine blood transfusion: Current indications and associated risks. Fetal Diagn. Ther. 2014, 36, 263–271. [Google Scholar] [CrossRef]
- Ree, I.M.C.; Smits-Wintjens, V.E.H.J.; van der Bom, J.G.; van Klink, J.M.M.; Oepkes, D.; Lopriore, E. Neonatal management and outcome in alloimmune hemolytic disease. Expert. Rev. Hematol. 2017, 10, 607–616. [Google Scholar] [CrossRef]
- Smits-Wintjens, V.E.; Rath, M.E.; Lindenburg, I.T.; Oepkes, D.; van Zwet, E.W.; Walther, F.J.; Lopriore, E. Cholestasis in neonates with red cell alloimmune hemolytic disease: Incidence, risk factors and outcome. Neonatology 2012, 101, 306–310. [Google Scholar] [CrossRef]
- Fawaz, R.; Baumann, U.; Ekong, U.; Fischler, B.; Hadzic, N.; Mack, C.L.; McLin, V.A.; Molleston, J.P.; Neimark, E.; Ng, V.L.; et al. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 154–168. [Google Scholar]
- Jankowska, I.; Kamińska, D.; Liberek, A.; Socha, P. Recommendation of early diagnosis of cholestasis in infant. Stand. Med. Pediatr. 2017, 14, 7–15. [Google Scholar]
- Lindenburg, I.T.; Smits-Wintjens, V.E.; van Klink, J.M.; Verduin, E.; van Kamp, I.L.; Walther, F.J.; Schonewille, H.; Doxiadis, I.I.; Kanhai, H.H.; van Lith, J.M.; et al. Long-term neurodevelopmental outcome after intrauterine transfusion for hemolytic disease of the fetus/newborn: The LOTUS study. Am. J. Obstet. Gynecol. 2012, 206, 141.e1–141.e8. [Google Scholar] [CrossRef]
- Maisels, M.J.; Watchko, J.F.; Bhutani, V.K.; Stevenson, D.K. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J. Perinatol. 2012, 32, 660–664. [Google Scholar] [CrossRef]
- Bhutani, V.K.; Wong, R.J.; Stevenson, D.K. Hyperbilirubinemia in Preterm Neonates. Clin. Perinatol. 2016, 43, 215–232. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Newman, T.B.; Slaughter, J.L.; Maisels, M.J.; Watchko, J.F.; Downs, S.M.; Grout, R.W.; Bundy, D.G.; Stark, A.R.; Bogen, D.L.; et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2022, 150, e2022058859. [Google Scholar] [CrossRef]
- Wallenstein, M.B.; Bhutani, V.K. Jaundice and kernicterus in the moderately preterm infant. Clin. Perinatol. 2013, 40, 679–688. [Google Scholar] [CrossRef]
- Smits-Wintjens, V.E.; Rath, M.E.; van Zwet, E.W.; Oepkes, D.; Brand, A.; Walther, F.J.; Lopriore, E. Neonatal morbidity after exchange transfusion for red cell alloimmune hemolytic disease. Neonatology 2013, 103, 141–147. [Google Scholar] [CrossRef]
- Teng, J.; Wickman, L.; Reilly, M.; Nemeth, A.; Fischler, B.; Bohlin, K.; Tiblad, E. Population-based incidence and risk factors for cholestasis in hemolytic disease of the fetus and newborn. J. Perinatol. 2022, 42, 702–707. [Google Scholar] [CrossRef]
- Rath, M.E.A.; Smits-Wintjens, V.E.H.J.; Oepkes, D.; Walther, F.J.; Lopriore, E. Iron status in infants with alloimmune haemolytic disease in the first three months of life. Vox Sang. 2013, 105, 328–333. [Google Scholar] [CrossRef]
- Demircioğlu, F.; Çağlayan Sözmen, Ş.; Yılmaz, Ş.; Ören, H.; Arslan, N.; Kumral, A.; Özer, E.; İrken, G. Severe iron overload and hyporegenerative anemia in a case with rhesus hemolytic disease: Therapeutic approach to rare complications. Turk. J. Haematol. 2010, 27, 204–208. [Google Scholar] [CrossRef]
- Zonneveld, R.; van der Meer-Kapelle, L.; Sylva, M.; Brand, A.; Zijlstra, M.; Schonewille, H. Severe Fetal Hemolysis and Cholestasis Due to High-Titer Maternal IgG Anti-A Antibodies. Pediatrics 2019, 143, e20182859. [Google Scholar] [CrossRef]
- Khdair-Ahmad, F.; Aladily, T.; Khdair-Ahmad, O.; Badran, E.F. Chelation therapy for secondary neonatal iron over load: Lessons learned from rhesus hemolytic disease. Turk. J. Pediatr. 2018, 60, 335–339. [Google Scholar] [CrossRef]
- Yilmaz, S.; Duman, N.; Ozer, E.; Kavas, N.; Oren, H.; Demircioğlu, F.; Kumral, A.; Ozkan, H.; Irken, G.; Ozer, E. A case of rhesus hemolytic disease with hemophagocytosis and severe iron overload due to multiple transfusions. J. Pediatr. Hematol. Oncol. 2006, 28, 290–292. [Google Scholar] [CrossRef]
- Sreenan, C.; Idikio, H.A.; Osiovich, H. Successful chelation therapy in a case of neonatal iron overload following intravascular intrauterine transfusion. J. Perinatol. 2000, 20 Pt 1, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Yalaz, M.; Bilgin, B.S.; Köroğlu, O.A.; Ay, Y.; Arıkan, C.; Sagol, S.; Akısü, M.; Kültürsay, N. Desferrioxamine treatment of iron overload secondary to RH isoimmunization and intrauterine transfusion in a newborn infant. Eur. J. Pediatr. 2011, 170, 1457–1460. [Google Scholar] [CrossRef]
- Drozdowska-Szymczak, A.; Proczka, J.; Chrzanowska-Liszewska, D.; Truszkowski, K.; Mazanowska, N.; Krajewski, P. Liver Dysfunction with Severe Cholestasis and Coagulation Disorders in the Course of Hemolytic Disease of the Newborn Requiring Chelation Therapy-A Case Report and Review of the Literature. J. Clin. Med. 2023, 12, 7645. [Google Scholar] [CrossRef]
- Grobler, J.M.; Mercer, M.J. Kernicterus associated with elevated predominantly direct-reacting bilirubin. South Afr. Med. J. 1997, 87, 1146. [Google Scholar]
- Watchko, J.F.; Maisels, M.J. Management of severe hyperbilirubinemia in the cholestatic neonate: A review and an approach. J. Perinatol. 2022, 42, 695–701. [Google Scholar] [CrossRef]
- Shamir, S.B.; Kurian, J.; Kogan-Liberman, D.; Taragin, B.H. Hepatic Imaging in Neonates and Young Infants: State of the Art. Radiology 2017, 285, 763–777. [Google Scholar] [CrossRef]
- Feldman, A.G.; Sokol, R.J. Neonatal Cholestasis. Neoreviews 2013, 14, e63–e73. [Google Scholar] [CrossRef]
- Jankowska, I.; Pawłowska, J.; Bakuła, A.; Czubkowski, P.; Gliwicz, D.; Teisseyre, M.; Socha, P. Management of cholestasis in infants. Stand. Med. Pediatr. 2013, 1, 41–48. [Google Scholar]


| TWIN A | TWIN B | ||||||
|---|---|---|---|---|---|---|---|
| Hgb (g/dL) | Hct (%) | RBC (1 × 1012/L) | Hgb (g/dL) | Hct (%) | RBC (1 × 1012/L) | ||
| IUT 1 | Before | 9.1 | 26.5 | 2.09 | 8.9 | 25.8 | 2.07 |
| After | 12.4 | 35.5 | 3.36 | 13.1 | 38.0 | 3.69 | |
| IUT 2 | Before | 8.2 | 21.4 | 1.95 | 9.1 | 23.9 | 2.21 |
| After | 12.6 | 33.8 | 3.51 | 12.8 | 35.1 | 3.55 | |
| Twin A | Twin B | |
|---|---|---|
| Number of intrauterine transfusions | 2 1 | 2 1 |
| Number of top-up transfusions | 3 2 | 2 3 |
| Number of exchange transfusions | 0 | 0 |
| Duration of phototherapy in days | 4 | 4 |
| Number of intravenous immunoglobulin infusions | 1 4 | 1 4 |
| Days of parenteral nutrition | 21 | 26 |
| Days of parenteral nutrition without enteral feedings | 8 | 12 |
| Duration of antibiotic therapy in days | 13 | 18 |
| Length of stay in days | 64 5 | 64 5 |
| Twin A | Twin B | |
|---|---|---|
| Initial hemoglobin (g/dL) | 8.1 | 9.7 |
| Lowest hemoglobin (g/dL) | 7.8 | 9.5 |
| Initial total bilirubin (mg/dL) | 6.7 | 9.9 |
| Highest total bilirubin (mg/dL) (Age in days) | 36.33 (7) | 50.9 (18) |
| Total bilirubin at discharge (mg/dL) | 1.99 | 1.99 |
| Initial direct bilirubin (mg/dL) | 1.94 | 5.51 |
| Highest direct bilirubin (mg/dL) (Age in days) | 32.21 (7) | 50.2 (18) |
| Direct bilirubin at discharge (mg/dL) | 1.87 | 1.88 |
| Aspartate transaminase (AST) (U/L) (Age in days) | 582 (14) | 595 (29) |
| Alanine transaminase (ALT) (U/L) (Age in days) | 607 (23) | 366 (29) |
| Gamma-glutamyl transpeptidase (GGT) (U/L) (Age in days) | 295 (19) | 245 (29) |
| Lowest total protein (g/dL) | 3.3 | 3.78 |
| Lowest albumin (g/dL) | 2.0 | 2.2 |
| Initial ferritin (ng/mL) | 932.5 | >1650 * |
| Highest ferritin (ng/mL) (Age in days) | 5281.76 * (57) | 5839.65 * (57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdowska-Szymczak, A.; Proczka, J.; Mazanowska, N.; Ludwin, A.; Krajewski, P. Severe Cholestasis in Neonates with Hemolytic Disease of the Fetus and Newborn—A Case Report. J. Clin. Med. 2024, 13, 1272. https://doi.org/10.3390/jcm13051272
Drozdowska-Szymczak A, Proczka J, Mazanowska N, Ludwin A, Krajewski P. Severe Cholestasis in Neonates with Hemolytic Disease of the Fetus and Newborn—A Case Report. Journal of Clinical Medicine. 2024; 13(5):1272. https://doi.org/10.3390/jcm13051272
Chicago/Turabian StyleDrozdowska-Szymczak, Agnieszka, Julia Proczka, Natalia Mazanowska, Artur Ludwin, and Paweł Krajewski. 2024. "Severe Cholestasis in Neonates with Hemolytic Disease of the Fetus and Newborn—A Case Report" Journal of Clinical Medicine 13, no. 5: 1272. https://doi.org/10.3390/jcm13051272