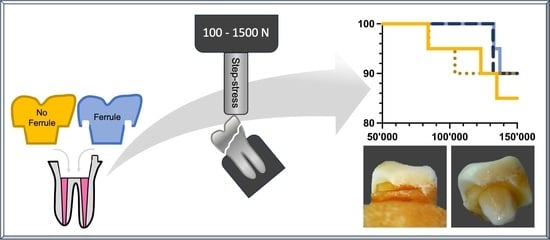
Influence of Cementation Mode and Ferrule Design on the Fatigue Resistance of Monolithic Zirconia Endocrowns
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al2O3 | Aluminum oxide |
| CEJ | Cemento-enamel junction |
| CEREC | Ceramic Reconstruction |
| FA | Ferrule, adhesive luting |
| FC | Ferrule, conventional cementation |
| GIC | Glass-ionomer cement |
| NaCl | Sodium chloride |
| NaN3 | Sodium azide |
| NFA | No ferrule, adhesive luting |
| NFC | No ferrule, conventional cementation |
References
- Zhu, Z.; Dong, X.Y.; He, S.; Pan, X.; Tang, L. Effect of Post Placement on the Restoration of Endodontically Treated Teeth: A Systematic Review. Int. J. Prosthodont. 2015, 5, 475–483. [Google Scholar] [CrossRef]
- Zarow, M.; Devoto, W.; Saracinelli, M. Reconstruction of endodontically treated posterior teeth with or without post? guidelines for the dental practitioner. Eur. J. Esthet. Dent. 2009, 4, 312–327. [Google Scholar] [PubMed]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature, Part II (Evaluation of fatigue behavior, interfaces, and in vivo studies). Quintessence Int. 2008, 39, 117–129. [Google Scholar]
- Tang, W.; Wu, Y.; Smales, R.J. Identifying and reducing risks for potential fractures in endodontically treated teeth. J. Endod. 2010, 36, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Schmitt de Andrade, G.; de Siqueira Ferreira Anzaloni Saavedra, G.; Gullo Augusto, M.; Alfonzo Leon, G.; Budel Brandão, H.C.; Mendes Tribst, J.P.; de Oliveira Dal Piva, A.M. Post-endodontic restorative treatments and their mechanical behavior: A narrative review. Dent. Rev. 2023, 3, 100067. [Google Scholar] [CrossRef]
- Soares, C.J.; Santana, F.R.; Silva, N.R.; Preira, J.C.; Pereira, C.A. Influence of the endodontic treatment on mechanical properties of root dentin. J. Endod. 2007, 33, 603–606. [Google Scholar] [CrossRef]
- Biacchi, G.R.; Mello, B.; Bastings, R.Z. The endocrown: An alternative approach for restoring extensively damaged molars. J. Esthet. Restor. Dent. 2013, 25, 383–391. [Google Scholar] [CrossRef]
- Govare, N.; Contrepois, M. Endocrowns: A systematic review. J. Prosthet. Dent. 2020, 3, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Monoblocks in root canals: A hypothetical or a tangible goal. J. Endod. 2007, 4, 391–398. [Google Scholar] [CrossRef]
- Zicari, F.; Van Meerbeek, B.; Scotti, R.; Naert, I. Effect of fibre post length and adhesive strategy on fracture resistance of endodontically treated teeth after fatigue loading. J. Dent. 2012, 4, 312–321. [Google Scholar] [CrossRef]
- Hatta, M.; Shinya, A.; Vallittu, P.K.; Shinya, A.; Lassila, L.V. High volume individual fibre post versus low volume fibre post: The fracture load of the restored tooth. J. Dent. 2011, 1, 65–71. [Google Scholar] [CrossRef]
- Falacho, R.I.; Melo, E.A.; Marques, J.A.; Ramos, J.C.; Guerra, F.; Blatz, M.B. Clinical in-situ evaluation of the effect of rubber dam isolation on bond strength to enamel. J. Esthet. Restor. Dent. 2023, 1, 48–55. [Google Scholar] [CrossRef]
- Miao, C.; Yang, X.; Wong, M.C.; Zou, J.; Zhou, X.; Li, C.; Wang, Y. Rubber dam isolation for restorative treatment in dental patients. Cochrane Database Syst. Rev. 2021, 5, CD009858. [Google Scholar] [CrossRef]
- Gargari, M.; Gloria, F.; Napoli, E.; Pujia, A.M. Zirconia: Cementation of prosthetic restorations. Literature review. Oral. Implantol. 2010, 4, 25–29. [Google Scholar]
- Segarra, M.; Segarra, A.A. The evolution of cements for indirect restorations from luting to bonding. In Practical Clinical Guide to Resin Cements, 1st ed.; Springer: Berlin, Germany, 2015; pp. 3–7. [Google Scholar]
- Nemane, V.; Akulwar, R.S.; Meshram, S. The Effect of Various Finish Line Configurations on the Marginal Seal and Occlusal Discrepancy of Cast Full Crowns After Cementation—An In-vitro Study. J. Clin. Diagn. Res. 2015, 8, 18–21. [Google Scholar] [CrossRef]
- Caplan, D.J.; Cai, J.; Yin, G.; White, B.A. Root canal filled versus non-root canal filled teeth: A retrospective comparison of survival times. J. Public Health Dent. 2005, 2, 90–96. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Robbins, J.W. Post placement and restoration of endodontically treated teeth: A literature review. J. Endod. 2004, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Al-Omiri, M.K.; Mahmoud, A.A.; Rayyan, M.R.; Abu-Hammad, O. Fracture resistance of teeth restored with post-retained restorations: An overview. J. Endod. 2010, 9, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lim, H.P.; Yang, H.S.; Park, S.W. Effect of ferrule on the fracture resistance of mandibular premolars with prefabricated posts and cores. J. Adv. Prosthodont. 2017, 5, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Samran, A.; El Bahra, S.; Kern, M. The influence of substance loss and ferrule height on the fracture resistance of endodontically treated premolars. An in vitro study. Dent. Mater. 2013, 12, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Jotkowitz, A.; Samet, N. Rethinking ferrule-a new approach to an old dilemma. Br. Dent. J. 2010, 1, 25–33. [Google Scholar] [CrossRef]
- Clark, D.; Khademi, J. Modern molar endodontic access and directed dentin conservation. Dent. Clin. N. Am. 2010, 2, 249–273. [Google Scholar] [CrossRef]
- Isidor, F.; Brøndum, K.; Ravnholt, G. The influence of post length and crown ferrule length on the resistance to cyclic loading of bovine teeth with prefabricated titanium posts International. J. Prosth 1999, 1, 78–82. [Google Scholar]
- Libman, W.J.; Nicholls, J.I. Load fatigue of teeth restored with cast posts and cores and complete crowns. Int. J. Prosthodont. 1995, 2, 155–161. [Google Scholar]
- Stankiewicz, N.R.; Wilson, P.R. The ferrule effect: A literature review. Int. Endod. J. 2002, 7, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Akkayan, B. An in vitro study evaluating the effect of ferrule length on fracture resistance of endodontically treated teeth restored with fiber-reinforced and zirconia dowel systems. J. Prosth Dent. 2004, 2, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.S.; Allahyari, S.; Niakan, S.; Atri, F. Effect of Preparation Design on Marginal Integrity and Fracture Resistance of Endocrowns: A Systematic Review. Front. Dent. 2022, 19, 37–38. [Google Scholar] [CrossRef]
- Einhorn, M.; DuVall, N.; Wajdowicz, M.; Brewster, J.; Roberts, H. Preparation Ferrule Design Effect on Endocrown Failure Resistance. J. Prosthodont. 2019, 1, 237–242. [Google Scholar] [CrossRef]
- Taha, D.; Spintzyk, S.; Schille, C.; Sabet, A.; Wahsh, M.; Salah, T. Fracture resistance and failure modes of polymer infiltrated ceramic endocrown restorations with variations in margin design and occlusal thickness. J. Prosthodont. Res. 2018, 3, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Bamajboor, A.; Dudley, J. The Influence of Ferrule on the Marginal Gap and Fracture Resistance of Zirconia Endo-crowns. Int. J. Prosthodont. 2022, 4, 494–501. [Google Scholar] [CrossRef]
- Fages, M.; Bennasar, B. The endocrown: A different type of all-ceramic reconstruction for molars. J. Can. Dent. Assoc. 2013, 79, 140–141. [Google Scholar]
- Alberto, L.H.J.; Zhang, Z.; Duan, Y. Effect of Ferrule Design on Stress Distribution of Maxillary Incisor Rehabilitated with Ceramic Crown and PEEK Post–Core Material: A 3D Finite Element Analysis. Ceramics 2023, 6, 2256–2268. [Google Scholar] [CrossRef]
- Rocca, G.T.; Daher, R.; Saratti, C.M.; Sedlacek, R.; Suchy, T.; Feilzer, A.J.; Krejci, I. Restoration of severely damaged endodontically treated premolars: The influence of the endo-core length on marginal integrity and fatigue resistance of lithium disilicate CAD-CAM ceramic endocrowns. J. Dent. 2018, 68, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Turkistani, A.A.; Dimashkieh, M.; Rayyan, M. Fracture resistance of teeth restored with endocrowns: An in vitro study. J. Esthet. Restor. Dent. 2020, 4, 389–394. [Google Scholar] [CrossRef] [PubMed]
- El-Damanhoury, H.M.; Haj-Ali, R.N.; Platt, J.A. Fracture resistance and microleakage of endocrowns utilizing three CAD-CAM blocks. Oper. Dent. 2015, 2, 201–210. [Google Scholar] [CrossRef]
- Hondrun, S.O. A review of the strength properties of dental ceramics. J. Prosth dent 1992, 76, 859–865. [Google Scholar] [CrossRef]
- Wiskott, H.W.; Nicholls, J.I.; Belser, U.C. Stress fatigue: Basic principles and prosthodontic implications. Int. J. Prosthodont. 1995, 2, 105–116. [Google Scholar]
- Gonzaga, C.C.; Cesar, P.F.; Miranda, W.G.; Yoshimura, H.N. Slow crack growth and reliability of dental ceramics. Dent. Mater. 2011, 4, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Bruzi, G.; Giannini, M.; Magne, P. Fatigue resistance of CAD/CAM complete crowns with a simplified cementation process. J. Prosthet. Dent. 2014, 4, 310–317. [Google Scholar] [CrossRef]
- Pires, C.W.; Lenzi, T.L.; Soares, F.Z.M.; Rocha, R.O. Zinc oxide eugenol paste jeopardises the adhesive bonding to primary dentine. Eur. Arch. Paediatr. Dent. 2018, 3, 163–169. [Google Scholar] [CrossRef]
- Wongsorachai, R.N.; Thanatvarakorn, O.; Prasansuttiporn, T.; Jittidecharaks, S.; Hosaka, K.; Foxton, R.M.; Nakajima, M. Effect of Polymerization Accelerator on Bond Strength to Eugenol-Contaminated Dentin. J. Adhes. Dent. 2018, 6, 541–547. [Google Scholar] [CrossRef]
- Lander, E.; Dietschi, D. Endocrowns: A clinical report. Quintessence Int. 2008, 39, 99–106. [Google Scholar]
- Stawarczyk, B.; Stich, N.; Eichberger, M.; Edelhoff, D.; Roos, M.; Gernet, W.; Keul, C. Long-term tensile bond strength of differently cemented nanocomposite CAD/CAM crowns on dentin abutment. Dent. Mater. 2014, 3, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, M.H.; Bahrami, G.; Finlay, S.; Isidor, F. Comparison of fatigue resistance and failure modes between metal-ceramic and all-ceramic crowns by cyclic loading in water. J. Dent. 2014, 12, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Cesar, P.F.; Scherrer, S.S.; Della Bona, A.; Van Noort, R.; Tholey, M.; Vichi, A.; Lohbauer, U. ADM guidance-ceramics: Fatigue principles and testing. Dent. Mater. 2017, 33, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Karakis, D.; Dogan, A. The craniofacial morphology and maximum bite force in sleep bruxism patients with signs and symptoms of temporomandibular disorders. Cranio 2015, 33, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Calderon, P.d.S.; Kogawa, E.M.; Lauris, J.R.; Conti, P.C. The influence of gender and bruxism on the human maxi-mum bite force. J. Appl. Oral. Sci. 2006, 14, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Ung, B.; Kettering, J.D. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J. Endod. 1990, 16, 566–569. [Google Scholar] [CrossRef]
- Dejak, B.; Młotkowski, A. 3D-Finite element analysis of molars restored with endocrowns and posts during masticatory simulation. Dent. Mater. 2013, 29, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bindl, A.; Richter, B.; Mormann, W.H. Survival of ceramic computer-aided design/manufacturing crowns bonded to preparations with reduced macroretention geometry. Int. J. Prosthodont. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Ahmed, M.A.A.; Kern, M.; Mourshed, B.; Wille, S.; Chaar, M.S. Fracture resistance of maxillary premolars restored with different endocrown designs and materials after artificial ageing. J. Prosthodont. Res. 2022, 1, 141–150. [Google Scholar] [CrossRef]
- Thomas, R.M.; Kelly, A.; Tagiyeva, N.; Kanagasingam, S. Comparing endocrown restorations on permanent molars and premolars: A systematic review and meta-analysis. Br. Dent. J. 2020; Online ahead of print. [Google Scholar] [CrossRef]
- Coelho, P.; Bromage, T.G. The Challenges in Engineering and Testing Dental Bioceramics. In High Strength Ceramics: Interdisciplinary Perspectives, 1st ed.; Ferencz, J.L., Silva, N.R.F.A., Navarro, J.M., Eds.; Quintessence Publishing: New York, NY, USA, 2014; pp. 1–16. [Google Scholar]
- Schönhoff, L.M.; Lümkemann, N.; Buser, R.; Hampe, R.; Stawarczyk, B. Fatigue resistance of monolithic strength-gradient zirconia materials. J. Mech. Behav. Biomed. Mater. 2021, 119, 104504. [Google Scholar] [CrossRef]
- Weigl, P.; Sander, A.; Wu, Y.; Felber, R.; Lauer, H.C.; Rosentritt, M. In-vitro performance and fracture strength of thin monolithic zirconia crowns. J. Adv. Prosthodont. 2018, 2, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, S.Y.; Bae, J.H.; Bae, E.B.; Huh, J.B. In vitro study of the fracture resistance of monolithic lithium disilicate, monolithic zirconia, and lithium disilicate pressed on zirconia for three-unit fixed dental prostheses. J. Adv. Prosthodont. 2017, 4, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Borba, M.; Cesar, P.F.; Griggs, J.A.; Della Bona, A. Step-stress analysis for predicting dental ceramic reliability. Dent. Mater. 2013, 8, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Venturini, A.B.; Bohrer, T.C.; Fontana, P.E.; Fröhlich, T.T.; May, L.G.; Valandro, L.F.; Step-stress, vs. staircase fatigue tests to evaluate the effect of intaglio adjustment on the fatigue behavior of simplified lithium disilicate glass-ceramic restorations. J. Mech. Behav. Biomed. Mater. 2021, 113, 104091. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Zanotti, G.; Tartaglia, G.M. Maximal bite forces in healthy young adults as predicted by surface electromyography. J. Dent. 2004, 32, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Pizolato, R.A.; Gavião, M.B.; Berretin-Felix, G.; Sampaio, A.C.; Trindade Junior, A.S. Maximal bite force in young adults with temporomandibular disorders and bruxism. Braz. Oral. Res. 2007, 21, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Takaki, P.; Vieira, M.; Bommarito, S. Maximum bite force analysis in different age groups. Int. Arch. Otorhinolaryngol. 2014, 18, 272–276. [Google Scholar] [CrossRef]
- Magne, P.; Carvalho, A.O.; Bruzi, G.; Anderson, R.E.; Maia, H.P.; Giannini, M. Influence of no-ferrule and no-post buildup design on the fatigue resistance of endodontically treated molars restored with resin nanoceramic CAD/CAM crowns. Oper. Dent. 2014, 39, 595–602. [Google Scholar] [CrossRef]
- Gresnight, M.M.M.; Özcan, M.; Van de Houten, M.L.A.; Schipper, L.; Cune, M.S. Fracture strength, failure type and Weibull characteristics of lithium disilicate and multiphase resin composite endocrowns under axial and lateral forces. Dent. Mater. 2016, 32, 607–614. [Google Scholar] [CrossRef]
- Lin, C.L.; Chang, Y.H.; Chang, C.Y.; Pai, C.A.; Huang, S.F. Finite element analyses to estimate failure risks in the ceramic endocrown and classical crown for endodontically treated maxillary premolars. Eur. J. Oral. Sci. 2010, 118, 87–93. [Google Scholar] [CrossRef]
- AboElhassan, R.G.; Watts, D.C.; Alamoush, R.A.; Elraggal, A. Biomechanical behavior and Weibull survival of CAD-CAM endocrowns with different marginal designs: A 3D finite element analysis. Dent. Mater. 2023, 23, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.P.; Weber, A.L.; Severo Bde, P.; Souza, M.A.; Cecchin, D. Effect of length post and remaining root tissue on fracture resistance of fibre posts relined with resin composite. J. Oral. Rehab 2015, 3, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Gregor, L.; Bouillaguet, S.; Onisor, I.; Ardu, S.; Krejci, I.; Rocca, G.T. Microhardness of light- and dual-polymerizable luting resins polymerized through 7.5-mm-thick endocrowns. J. Prosthet. Dent. 2014, 4, 942–948. [Google Scholar] [CrossRef]
- Goldman, A.S.; Chen, X.; Fan, M.; Frencken, J.E. Cost-effectiveness, in a randomized trial, of glass-ionomer-based and resin sealant materials after 4 yr. Eur. J. Oral. Sci. 2016, 5, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Tuygunov, N.; Zakaria, M.N.; Yahya, N.A.; Abdul Aziz, A.; Cahyanto, A. Efficacy and bone-contact biocompatibility of glass ionomer cement as a biomaterial for bone regeneration: A systematic review. J. Mech. Behav. Biomed. Mater. 2023, 146, 106099. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.R.; Richards, M.W.; Meiers, J.C. Early erosion of glass-ionomer cement at crown margins. Int. J. Prosthodont. 1993, 6, 553–557. [Google Scholar]
- El Ghoul, W.; Ozcan, M.; Silwadi, M.; Salameh, Z. Fracture resistance and failure modes of endocrowns manufactured with different CAD-CAM materials under axial and lateral loading. J. Esthet. Restor. Dent. 2019, 31, 378–387. [Google Scholar] [CrossRef]
- Lise, D.P.; Van Ende, A.; De Munck, J.; Suzuki, T.Y.; Vieira, L.C.; Van Meerbeek, B. Biomechanical behavior of endodontically treated premolars using different preparation designs and CAD-CAM materials. J. Dent. 2017, 59, 54–61. [Google Scholar] [CrossRef]
- Lad, P.P.; Kamath, M.; Tarale, K.; Kusugal, P.B. Practical clinical considerations of luting cements: A review. J. Int. Oral. Health 2014, 6, 116–120. [Google Scholar] [PubMed]
- Al-Dabbagh, R.A. Survival and success of endocrowns: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 3, 415–416. [Google Scholar] [CrossRef] [PubMed]








| Group (n = 20) Total Failures (Percentage) | Non Restorable | Restorable | Cycles | Load (N) |
|---|---|---|---|---|
| NFC 3 (15%) | 1 | 83,950 | 600 N | |
| 1 | 123,021 | 1300 N | ||
| 1 | 134,925 | 1400 N | ||
| NFA 2 (10%) | 1 | 84,433 | 700 N | |
| 1 | 103,975 | 900 N | ||
| FC 2 (10%) | 1 | 137,112 | 1400 N | |
| 1 | 132,650 | 1400 N | ||
| FA 2 (10%) | 1 | 132,211 | 1400 N | |
| 1 | 132,112 | 1400 N |
| Group (n = 20) | Total Failures | Non-Restorable | Restorable | Cohesive Failure (Tooth) | Cohesive Failure (Restauration) | Combined Failure | Loss of Retention |
|---|---|---|---|---|---|---|---|
| NFC | 3 (15%) | 3 | 3 | / | / | ||
| NFA | 2 (10%) | 1 | 1 | 2 | / | / | |
| FC | 2 (10%) | 1 | 1 | 1 | / | 1 | / |
| FA | 2 (10%) | 1 | 1 | 1 | / | 1 | / |
| Log-Rank Test | NFA | FC |
|---|---|---|
| NFC | p = 0.6551 | p = 0.6209 |
| FA | p = 0.9580 | p = 0.9580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoilov, M.; Boehmer, T.; Stoilov, L.; Stark, H.; Marder, M.; Enkling, N.; Kraus, D. Influence of Cementation Mode and Ferrule Design on the Fatigue Resistance of Monolithic Zirconia Endocrowns. J. Clin. Med. 2024, 13, 1165. https://doi.org/10.3390/jcm13041165
Stoilov M, Boehmer T, Stoilov L, Stark H, Marder M, Enkling N, Kraus D. Influence of Cementation Mode and Ferrule Design on the Fatigue Resistance of Monolithic Zirconia Endocrowns. Journal of Clinical Medicine. 2024; 13(4):1165. https://doi.org/10.3390/jcm13041165
Chicago/Turabian StyleStoilov, Milan, Tobias Boehmer, Lea Stoilov, Helmut Stark, Michael Marder, Norbert Enkling, and Dominik Kraus. 2024. "Influence of Cementation Mode and Ferrule Design on the Fatigue Resistance of Monolithic Zirconia Endocrowns" Journal of Clinical Medicine 13, no. 4: 1165. https://doi.org/10.3390/jcm13041165