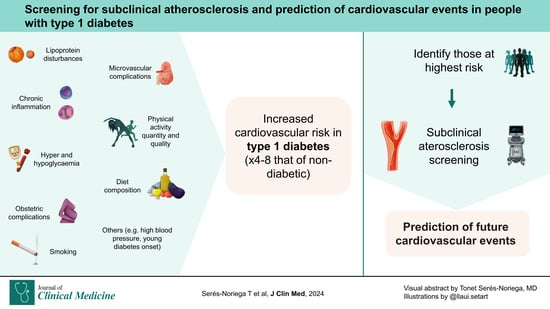
Screening for Subclinical Atherosclerosis and the Prediction of Cardiovascular Events in People with Type 1 Diabetes
Abstract
:1. Introduction
2. Methods of Searching
3. Physiopathology of Atherosclerosis in Diabetes
4. Screening Methods for Subclinical Atherosclerosis
4.1. Carotid Ultrasound
4.2. Coronary Artery Calcium (CAC) Scan
4.3. Coronary Computed Tomography Angiography (CCTA)
4.4. Ankle–Brachial Index (ABI)
4.5. Magnetic Resonance Imaging (MRI)
4.6. Other Screening Methods
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Statin Eligibility for Primary Prevention of Cardiovascular Disease According to 2021 European Prevention Guidelines Compared with Other International Guidelines. JAMA Cardiol. 2022, 7, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Damen, J.A.; Pajouheshnia, R.; Heus, P.; Moons, K.G.M.; Reitsma, J.B.; Scholten, R.J.P.M.; Hooft, L.; Debray, T.P.A. Performance of the Framingham Risk Models and Pooled Cohort Equations for Predicting 10-Year Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. BMC Med. 2019, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Kavousi, M.; Leening, M.J.G.; Nanchen, D.; Greenland, P.; Graham, I.M.; Steyerberg, E.W.; Ikram, M.A.; Stricker, B.H.; Hofman, A.; Franco, O.H. Comparison of Application of the ACC/AHA Guidelines, Adult Treatment Panel III Guidelines, and European Society of Cardiology Guidelines for Cardiovascular Disease Prevention in a European Cohort. JAMA 2014, 311, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Tota-Maharaj, R.; Blaha, M.J.; Blankstein, R.; Silverman, M.G.; Eng, J.; Shaw, L.J.; Blumenthal, R.S.; Budoff, M.J.; Nasir, K. Association of Coronary Artery Calcium and Coronary Heart Disease Events in Young and Elderly Participants in the Multi-Ethnic Study of Atherosclerosis: A Secondary Analysis of a Prospective, Population-Based Cohort. Mayo Clin. Proc. 2014, 89, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Nambi, V.; Chambless, L.; Folsom, A.R.; He, M.; Hu, Y.; Mosley, T.; Volcik, K.; Boerwinkle, E.; Ballantyne, C.M. Carotid Intima-Media Thickness and Presence or Absence of Plaque Improves Prediction of Coronary Heart Disease Risk: The ARIC (Atherosclerosis Risk In Communities) Study. J. Am. Coll. Cardiol. 2010, 55, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Den Ruijter, H.M.; Bots, M.L.; Moons, K.G.M. Improvements in Risk Stratification for the Occurrence of Cardiovascular Disease by Imaging Subclinical Atherosclerosis: A Systematic Review. Heart 2012, 98, 177–184. [Google Scholar] [CrossRef]
- Mendieta, G.; Pocock, S.; Mass, V.; Moreno, A.; Owen, R.; García-Lunar, I.; López-Melgar, B.; Fuster, J.J.; Andres, V.; Pérez-Herreras, C.; et al. Determinants of Progression and Regression of Subclinical Atherosclerosis Over 6 Years. J. Am. Coll. Cardiol. 2023, 82, 2069–2083. [Google Scholar] [CrossRef]
- Carr, J.J.; Jacobs, D.R.; Terry, J.G.; Shay, C.M.; Sidney, S.; Liu, K.; Schreiner, P.J.; Lewis, C.E.; Shikany, J.M.; Reis, J.P.; et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years with Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017, 2, 391–399. [Google Scholar] [CrossRef]
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.C.; et al. Effect of Very High-Intensity Statin Therapy on Regression of Coronary Atherosclerosis: The ASTEROID Trial. JAMA 2006, 295, 1556–1565. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; Van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice: Developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies with the Special Contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Näslund, U.; Ng, N.; Lundgren, A.; Fhärm, E.; Grönlund, C.; Johansson, H.; Lindahl, B.; Lindahl, B.; Lindvall, K.; Nilsson, S.K.; et al. Visualization of Asymptomatic Atherosclerotic Disease for Optimum Cardiovascular Prevention (VIPVIZA): A Pragmatic, Open-Label, Randomised Controlled Trial. Lancet 2019, 393, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef]
- Harjutsalo, V.; Pongrac Barlovic, D.; Groop, P.H. Long-Term Population-Based Trends in the Incidence of Cardiovascular Disease in Individuals with Type 1 Diabetes from Finland: A Retrospective, Nationwide, Cohort Study. Lancet Diabetes Endocrinol. 2021, 9, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Svensson, A.-M.; Kosiborod, M.; Gudbjörnsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic Control and Excess Mortality in Type 1 Diabetes. N. Engl. J. Med. 2014, 371, 1972–1982. [Google Scholar] [CrossRef]
- Vergès, B. Cardiovascular Disease in Type 1 Diabetes, an Underestimated Danger: Epidemiological and Pathophysiological Data. Atherosclerosis 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Sousa, G.R.; Pober, D.; Galderisi, A.; Lv, H.J.; Yu, L.; Pereira, A.C.; Doria, A.; Kosiborod, M.; Lipes, M.A. Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus. Circulation 2019, 139, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Yang, Z.; Lu, Y.; Zou, C. Causal Associations between Type 1 Diabetes Mellitus and Cardiovascular Diseases: A Mendelian Randomization Study. Cardiovasc. Diabetol. 2023, 22, 236. [Google Scholar] [CrossRef] [PubMed]
- Serés-Noriega, T.; Giménez, M.; Perea, V.; Boswell, L.; Viñals, C.; Blanco, J.; Vinagre, I.; Pané, A.; Esmatjes, E.; Conget, I.; et al. Use of the Steno T1 Risk Engine Identifies Preclinical Atherosclerosis Better Than Use of ESC/EASD-2019 in Adult Subjects with Type 1 Diabetes at High Risk. Diabetes Care 2022, 45, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- McGurnaghan, S.J.; McKeigue, P.M.; Read, S.H.; Franzen, S.; Svensson, A.M.; Colombo, M.; Livingstone, S.; Farran, B.; Caparrotta, T.M.; Blackbourn, L.A.K.; et al. Development and Validation of a Cardiovascular Risk Prediction Model in Type 1 Diabetes. Diabetologia 2021, 64, 2001–2011. [Google Scholar] [CrossRef]
- Vistisen, D.; Andersen, G.S.; Hansen, C.S.; Hulman, A.; Henriksen, J.E.; Bech-Nielsen, H.; Jørgensen, M.E. Prediction of First Cardiovascular Disease Event in Type 1 Diabetes Mellitus the Steno Type 1 Risk Engine. Circulation 2016, 133, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781. [Google Scholar] [CrossRef]
- Gudgeon, J.; Marín-Rubio, J.L.; Trost, M. The Role of Macrophage Scavenger Receptor 1 (MSR1) in Inflammatory Disorders and Cancer. Front. Immunol. 2022, 13, 1012002. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M. Glycation and Carboxymethyllysine Levels in Skin Collagen Predict the Risk of Future 10-Year Progression of Diabetic Retinopathy and Nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications Participants with Type 1 Diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Katakami, N.; Kuroda, A.; Takahara, M.; Sakamoto, F.; Kawamori, D.; Matsuoka, T.; Matsuhisa, M.; Shimomura, I. Skin Autofluorescence Is Associated with Early-Stage Atherosclerosis in Patients with Type 1 Diabetes. J. Atheroscler. Thromb. 2017, 24, 312. [Google Scholar] [CrossRef]
- Katakami, N.; Kaneto, H.; Hao, H.; Umayahara, Y.; Fujitani, Y.; Sakamoto, K.; Gorogawa, S.I.; Yasuda, T.; Kawamori, D.; Kajimoto, Y.; et al. Role of Pim-1 in Smooth Muscle Cell Proliferation. J. Biol. Chem. 2004, 279, 54742–54749. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247. [Google Scholar] [CrossRef]
- Mariaca, K.; Serés-Noriega, T.; Viñals, C.; Perea, V.; Conget, I.; Mesa, A.; Boswell, L.; Font, C.; Pané, A.; Vinagre, I.; et al. Neutrophil-to-Lymphocyte Ratio Is Independently Associated with Carotid Atherosclerosis Burden in Individuals with Type 1 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Serés-Noriega, T.; Giménez, M.; Perea, V.; Blanco, J.; Vinagre, I.; Pané, A.; Ruiz, S.; Cofán, M.; Mesa, A.; Esmatjes, E.; et al. Quantification of Glycoproteins by Nuclear Magnetic Resonance Associated with Preclinical Carotid Atherosclerosis in Patients with Type 1 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.M.; Van Heck, J.I.P.; Stienstra, R.; Aarntzen, E.H.J.G.; Van Diepen, J.A.; Riksen, N.P.; Tack, C.J. Arterial Wall Inflammation Assessed by 18F-FDG-PET/CT Is Higher in Individuals with Type 1 Diabetes and Associated with Circulating Inflammatory Proteins. Cardiovasc. Res. 2023, 119, 1942–1951. [Google Scholar] [CrossRef]
- Das Evcimen, N.; King, G.L. The Role of Protein Kinase C Activation and the Vascular Complications of Diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Amor, A.J.; Castelblanco, E.; Hernández, M.; Gimenez, M.; Granado-Casas, M.; Blanco, J.; Soldevila, B.; Esmatjes, E.; Conget, I.; Alonso, N.; et al. Advanced Lipoprotein Profile Disturbances in Type 1 Diabetes Mellitus: A Focus on LDL Particles. Cardiovasc. Diabetol. 2020, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Serés-Noriega, T.; Ortega, E.; Giménez, M.; Perea, V.; Boswell, L.; Mariaca, K.; Font, C.; Mesa, A.; Viñals, C.; Blanco, J.; et al. Advanced Lipoprotein Profile Identifies Atherosclerosis Better than Conventional Lipids in Type 1 Diabetes at High Cardiovascular Risk. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Dyslipidemia in Type 1 Diabetes: A Masked Danger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Y.Y.; Lv, T.T.; Guan, S.Y.; Li, X.M.; Li, X.P.; Pan, H.F. Subclinical Atherosclerosis in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Angiology 2018, 70, 141–159. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011). Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kopyto, E.; Czeczelewski, M.; Mikos, E.; Stępniak, K.; Kopyto, M.; Matuszek, M.; Nieoczym, K.; Czarnecki, A.; Kuczyńska, M.; Cheda, M.; et al. Contrast-Enhanced Ultrasound Feasibility in Assessing Carotid Plaque Vulnerability—Narrative Review. J. Clin. Med. 2023, 12, 6416. [Google Scholar] [CrossRef]
- López-Melgar, B.; Mass, V.; Nogales, P.; Sánchez-González, J.; Entrekin, R.; Collet-Billon, A.; Rossello, X.; Fernández-Friera, L.; Fernández-Ortiz, A.; Sanz, J.; et al. New 3-Dimensional Volumetric Ultrasound Method for Accurate Quantification of Atherosclerotic Plaque Volume. JACC Cardiovasc. Imaging 2022, 15, 1124–1135. [Google Scholar] [CrossRef]
- Gupta, A.; Kesavabhotla, K.; Baradaran, H.; Kamel, H.; Pandya, A.; Giambrone, A.E.; Wright, D.; Pain, K.J.; Mtui, E.E.; Suri, J.S.; et al. Plaque Echolucency and Stroke Risk in Asymptomatic Carotid Stenosis: A Systematic Review and Meta-Analysis. Stroke 2015, 46, 91–97. [Google Scholar] [CrossRef]
- Sultan, S.R.; Khayat, M.; Almutairi, B.; Marzouq, A.; Albngali, A.; Abdeen, R.; Alahmadi, A.A.S.; Toonsi, F. B-Mode Ultrasound Characteristics of Carotid Plaques in Symptomatic and Asymptomatic Patients with Low-Grade Stenosis. PLoS ONE 2023, 18, e0291450. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Backlund, J.Y.C.; Cleary, P.A.; Harrington, A.P.; O’Leary, D.H.; Lachin, J.M.; Nathan, D.M. Progression of Carotid Artery Intima-Media Thickness during 12 Years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2011, 60, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Backlund, J.Y.C.; Budoff, M.; Raskin, P.; Bebu, I.; Lachin, J.M. Coronary Artery Disease Events and Carotid Intima-Media Thickness in Type 1 Diabetes in the DCCT/EDIC Cohort. J. Am. Heart Assoc. 2021, 10, 22922. [Google Scholar] [CrossRef]
- Ahmadi, A.; Argulian, E.; Leipsic, J.; Newby, D.E.; Narula, J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1608–1617. [Google Scholar] [CrossRef]
- Maftei, O.; Pena, A.S.; Sullivan, T.; Jones, T.W.; Donaghue, K.C.; Cameron, F.J.; Davis, E.; Cotterill, A.; Craig, M.E.; Gent, R.; et al. Early Atherosclerosis Relates to Urinary Albumin Excretion and Cardiovascular Risk Factors in Adolescents with Type 1 Diabetes: Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT). Diabetes Care 2014, 37, 3069–3075. [Google Scholar] [CrossRef]
- Amor, A.J.; Vinagre, I.; Valverde, M.; Pané, A.; Urquizu, X.; Meler, E.; López, E.; Quirós, C.; Giménez, M.; Codina, L.; et al. Preeclampsia Is Associated with Increased Preclinical Carotid Atherosclerosis in Women with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 85–95. [Google Scholar] [CrossRef]
- Perea, V.; Vinagre, I.; Serés-Noriega, T.; Viñals, C.; Mesa, A.; Pané, A.; Milad, C.; Esmatjes, E.; Conget, I.; Giménez, M.; et al. Impact of Preeclampsia and Parity on Sex-Based Discrepancies in Subclinical Carotid Atherosclerosis in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2023, dgad755. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, M.; Castelblanco, E.; Valldeperas, X.; Betriu, À.; Traveset, A.; Granado-Casas, M.; Hernández, M.; Vázquez, F.; Martín, M.; Rubinat, E.; et al. Diabetic Retinopathy Is Associated with the Presence and Burden of Subclinical Carotid Atherosclerosis in Type 1 Diabetes. Cardiovasc. Diabetol. 2018, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.S.; Liew, G.; Anderson, J.; Giles, L.C.; Gent, R.; Wong, T.Y.; Couper, J.J. Early Atherosclerosis Is Associated with Retinal Microvascular Changes in Adolescents with Type 1 Diabetes. Pediatr. Diabetes 2018, 19, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Pané, A.; Conget, I.; Boswell, L.; Ruiz, S.; Viñals, C.; Perea, V.; Giménez, M.; Cofán, M.; Blanco, J.; Vinagre, I.; et al. Insulin Resistance Is Associated with Preclinical Carotid Atherosclerosis in Patients with Type 1 Diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3323. [Google Scholar] [CrossRef]
- Rathsman, B.; Rosfors, S.; Sjöholm, Å.; Nyström, T. Early Signs of Atherosclerosis Are Associated with Insulin Resistance in Non-Obese Adolescent and Young Adults with Type 1 Diabetes. Cardiovasc. Diabetol. 2012, 11, 145. [Google Scholar] [CrossRef]
- Purnell, J.Q.; John, E.H.; Cleary, P.A.; Nathan, D.M.; Lachin, J.M.; Zinman, B.; Brunzell, J.D. The Effect of Excess Weight Gain with Intensive Diabetes Mellitus Treatment on Cardiovascular Disease Risk Factors and Atherosclerosis in Type 1 Diabetes Mellitus: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) Study. Circulation 2013, 127, 180–187. [Google Scholar] [CrossRef]
- Chahal, H.; Backlund, J.Y.C.; Cleary, P.A.; Lachin, J.M.; Polak, J.F.; Lima, J.A.C.; Bluemke, D.A. Relation between Carotid Intima-Media Thickness and Left Ventricular Mass in Type 1 Diabetes Mellitus (from the Epidemiology of Diabetes Interventions and Complications [EDIC] Study). Am. J. Cardiol. 2012, 110, 1534–1540. [Google Scholar] [CrossRef]
- Inkeri, J.; Tynjälä, A.; Forsblom, C.; Liebkind, R.; Tatlisumak, T.; Thorn, L.M.; Groop, P.H.; Shams, S.; Putaala, J.; Martola, J.; et al. Carotid Intima-Media Thickness and Arterial Stiffness in Relation to Cerebral Small Vessel Disease in Neurologically Asymptomatic Individuals with Type 1 Diabetes. Acta Diabetol. 2021, 58, 929–937. [Google Scholar] [CrossRef]
- Van Duinkerken, E.; IJzerman, R.G.; van der Zijl, N.J.; Barkhof, F.; Pouwels, P.J.W.; Schoonheim, M.M.; Moll, A.C.; Boerop, J.; Wessels, A.M.; Klein, M.; et al. Differential Impact of Subclinical Carotid Artery Disease on Cerebral Structure and Functioning in Type 1 Diabetes Patients with versus Those without Proliferative Retinopathy. Cardiovasc. Diabetol. 2014, 13, 58. [Google Scholar] [CrossRef]
- Hunt, K.J.; Baker, N.L.; Cleary, P.A.; Klein, R.; Virella, G.; Lopes-Virella, M.F. Longitudinal Association Between Endothelial Dysfunction, Inflammation, and Clotting Biomarkers with Subclinical Atherosclerosis in Type 1 Diabetes: An Evaluation of the DCCT/EDIC Cohort. Diabetes Care 2015, 38, 1281–1289. [Google Scholar] [CrossRef]
- Polak, J.F.; Szklo, M.; Kronmal, R.A.; Burke, G.L.; Shea, S.; Zavodni, A.E.H.; O’Leary, D.H. The Value of Carotid Artery Plaque and Intima-Media Thickness for Incident Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000087. [Google Scholar] [CrossRef]
- Nathan, D.M.; Lachin, J.; Cleary, P.; Orchard, T.; Brillon, D.J.; Backlund, J.-Y.; O’Leary, D.H.; Genuth, S. Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group Intensive Diabetes Therapy and Carotid Intima-Media Thickness in Type 1 Diabetes Mellitus. N. Engl. J. Med. 2003, 348, 2294–2303. [Google Scholar] [CrossRef]
- Timmons, J.G.; Greenlaw, N.; Boyle, J.G.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; et al. Metformin and Carotid Intima-Media Thickness in Never-Smokers with Type 1 Diabetes: The REMOVAL Trial. Diabetes Obes. Metab. 2021, 23, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Schäfer, M.; Truong, U.; Cree-Green, M.; Pyle, L.; Baumgartner, A.; Reyes, Y.G.; Maniatis, A.; Nayak, S.; Wadwa, R.P.; et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth with Type 1 Diabetes Mellitus. Circulation 2018, 138, 2895–2907. [Google Scholar] [CrossRef] [PubMed]
- Boswell, L.; Serés-Noriega, T.; Mesa, A.; Perea, V.; Pané, A.; Viñals, C.; Blanco, J.; Giménez, M.; Vinagre, I.; Esmatjes, E.; et al. Carotid Ultrasonography as a Strategy to Optimize Cardiovascular Risk Management in Type 1 Diabetes: A Cohort Study. Acta Diabetol. 2022, 59, 1563–1574. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Shahraki, M.N.; Jouabadi, S.M.; Bos, D.; Stricker, B.H.; Ahmadizar, F. Statin Use and Coronary Artery Calcification: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Controlled Trials. Curr. Atheroscler. Rep. 2023, 25, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Houslay, E.S.; Cowell, S.J.; Prescott, R.J.; Reid, J.; Burton, J.; Northridge, D.B.; Boon, N.A.; Newby, D.E. Progressive Coronary Calcification despite Intensive Lipid-Lowering Treatment: A Randomised Controlled Trial. Heart 2006, 92, 1207–1212. [Google Scholar] [CrossRef]
- Razavi, A.C.; Agatston, A.S.; Shaw, L.J.; De Cecco, C.N.; van Assen, M.; Sperling, L.S.; Bittencourt, M.S.; Daubert, M.A.; Nasir, K.; Blumenthal, R.S.; et al. Evolving Role of Calcium Density in Coronary Artery Calcium Scoring and Atherosclerotic Cardiovascular Disease Risk. JACC Cardiovasc. Imaging 2022, 15, 1648–1662. [Google Scholar] [CrossRef]
- Thomas, I.C.; Shiau, B.; Denenberg, J.O.; McClelland, R.L.; Greenland, P.; De Boer, I.H.; Kestenbaum, B.R.; Lin, G.M.; Daniels, M.; Forbang, N.I.; et al. Association of Cardiovascular Disease Risk Factors with Coronary Artery Calcium Volume versus Density. Heart 2018, 104, 135–143. [Google Scholar] [CrossRef]
- Arad, Y.; Goodman, K.J.; Roth, M.; Newstein, D.; Guerci, A.D. Coronary Calcification, Coronary Disease Risk Factors, C-Reactive Protein, and Atherosclerotic Cardiovascular Disease Events: The St. Francis Heart Study. J. Am. Coll. Cardiol. 2005, 46, 158–165. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Budoff, M.; Backlund, J.Y.C.; Bluemke, D.A.; Polak, J.; Bebu, I.; Schade, D.; Strowig, S.; Raskin, P.; Lachin, J.M. The Association of Coronary Artery Calcification with Subsequent Incidence of Cardiovascular Disease in Type 1 Diabetes: The DCCT/EDIC Trials. JACC Cardiovasc. Imaging 2019, 12, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Erqou, S.A.; Miller, R.G.; Edmundowicz, D.; Orchard, T.J.; Costacou, T. The Role of Coronary Artery Calcification Testing in Incident Coronary Artery Disease Risk Prediction in Type 1 Diabetes. Diabetologia 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Hoffmann, U.; Massaro, J.M.; Fox, C.S.; Manders, E.; O’Donnell, C.J. Defining Normal Distributions of Coronary Artery Calcium in Women and Men (from the Framingham Heart Study). Am. J. Cardiol. 2008, 102, 1136–1141.e1. [Google Scholar] [CrossRef] [PubMed]
- Lovshin, J.A.; Bjornstad, P.; Lovblom, L.E.; Bai, J.W.; Lytvyn, Y.; Boulet, G.; Farooqi, M.A.; Santiago, S.; Orszag, A.; Scarr, D.; et al. Atherosclerosis and Microvascular Complications: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care 2018, 41, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Serra, E.; Granada, M.L.; Alonso, N.; Pellitero, S.; Pizarro, E.; Reverter, J.L.; Salinas, I.; Soldevila, B.; Mauricio, D.; et al. Low Prevalence of Subclinical Atherosclerosis in Asymptomatic Patients with Type 1 Diabetes in a European Mediterranean Population. Diabetes Care 2014, 37, 814–820. [Google Scholar] [CrossRef]
- Salem, M.; Moneir, I.; Adly, A.M.; Esmat, K. Study of Coronary Artery Calcification Risk in Egyptian Adolescents with Type-1 Diabetes. Acta Diabetol. 2011, 48, 41–53. [Google Scholar] [CrossRef]
- De Block, C.E.M.; Shivalkar, B.; Goovaerts, W.; Brits, T.; Carpentier, K.; Verrijken, A.; Van Hoof, V.; Parizel, P.M.; Vrints, C.; Van Gaal, L.F. Coronary Artery Calcifications and Diastolic Dysfunction versus Visceral Fat Area in Type 1 Diabetes: VISCERA Study. J. Diabetes Complicat. 2018, 32, 271–278. [Google Scholar] [CrossRef]
- Hjortkjær, H.; Jensen, T.; Hilsted, J.; Corinth, H.; Mogensen, U.M.; Køber, L.; Fuchs, A.; Nordestgaard, B.G.; Kofoed, K.F. Possible Early Detection of Coronary Artery Calcium Progression in Type 1 Diabetes: A Case-Control Study of Normoalbuminuric Type 1 Diabetes Patients and Matched Controls. Diabetes Res. Clin. Pract. 2018, 141, 18–25. [Google Scholar] [CrossRef]
- Keshawarz, A.; Pyle, L.; Alman, A.; Sassano, C.; Westfeldt, E.; Sippl, R.; Snell-Bergeon, J. Type 1 Diabetes Accelerates Progression of Coronary Artery Calcium Over the Menopausal Transition: The CACTI Study. Diabetes Care 2019, 42, 2315–2321. [Google Scholar] [CrossRef]
- Costacou, T.; Edmundowicz, D.; Prince, C.; Conway, B.; Orchard, T.J. Progression of Coronary Artery Calcium in Type 1 Diabetes Mellitus. Am. J. Cardiol. 2007, 100, 1543–1547. [Google Scholar] [CrossRef]
- Guo, J.; Nunley, K.A.; Costacou, T.; Miller, R.G.; Rosano, C.; Edmundowicz, D.; Orchard, T.J. Greater Progression of Coronary Artery Calcification Is Associated with Clinically Relevant Cognitive Impairment in Type 1 Diabetes. Atherosclerosis 2019, 280, 58–65. [Google Scholar] [CrossRef]
- Bjornstad, P.; Maahs, D.M.; Duca, L.M.; Pyle, L.; Rewers, M.; Johnson, R.J.; Snell-Bergeon, J.K. Estimated Insulin Sensitivity Predicts Incident Micro- and Macrovascular Complications in Adults with Type 1 Diabetes over 6 Years: The Coronary Artery Calcification in Type 1 Diabetes Study. J. Diabetes Complicat. 2016, 30, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; Jalal, D.; Chonchol, M.; Johnson, R.J.; Rewers, M.; Snell-Bergeon, J.K. Impaired Renal Function Further Increases Odds of 6-Year Coronary Artery Calcification Progression in Adults with Type 1 Diabetes: The CACTI Study. Diabetes Care 2013, 36, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Alman, A.C.; Kinney, G.L.; Tracy, R.P.; Maahs, D.M.; Hokanson, J.E.; Rewers, M.J.; Snell-Bergeon, J.K. Prospective Association between Inflammatory Markers and Progression of Coronary Artery Calcification in Adults with and without Type 1 Diabetes. Diabetes Care 2013, 36, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.C.; Veyna, A.M.; Haarhues, M.D.; Kinney, G.L.; Rewers, M.; Snell-Bergeon, J.K. Obesity and Coronary Artery Calcium in Diabetes: The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes Technol. Ther. 2011, 13, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Snell-Bergeon, J.K.; Hokanson, J.E.; Jensen, L.; MacKenzie, T.; Kinney, G.; Dabelea, D.; Eckel, R.H.; Ehrlich, J.; Garg, S.; Rewers, M. Progression of Coronary Artery Calcification in Type 1 Diabetes: The Importance of Glycemic Control. Diabetes Care 2003, 26, 2923–2928. [Google Scholar] [CrossRef]
- Cleary, P.A.; Orchard, T.J.; Genuth, S.; Wong, N.D.; Detrano, R.; Backlund, J.Y.C.; Zinman, B.; Jacobson, A.; Sun, W.; Lachin, J.M.; et al. The Effect of Intensive Glycemic Treatment on Coronary Artery Calcification in Type 1 Diabetic Participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006, 55, 3556. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary Atherosclerosis Imaging by Coronary CT Angiography: Current Status, Correlation with Intravascular Interrogation and Meta-Analysis. JACC Cardiovasc. Imaging 2011, 4, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.M.; Chen, M.Y.; Dey, A.K.; Virmani, R.; Finn, A.V.; Khamis, R.Y.; Choi, A.D.; Min, J.K.; Williams, M.C.; Buckler, A.J.; et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies. J. Am. Coll. Cardiol. 2020, 76, 1226–1243. [Google Scholar] [CrossRef]
- Jiang, S.; Fang, C.; Xu, X.; Xing, L.; Sun, S.; Peng, C.; Yin, Y.; Lei, F.; Wang, Y.; Li, L.; et al. Identification of High-Risk Coronary Lesions by 3-Vessel Optical Coherence Tomography. J. Am. Coll. Cardiol. 2023, 81, 1217–1230. [Google Scholar] [CrossRef]
- Pinilla-Echeverri, N.; Mehta, S.R.; Wang, J.; Lavi, S.; Schampaert, E.; Cantor, W.J.; Bainey, K.R.; Welsh, R.C.; Kassam, S.; Mehran, R.; et al. Nonculprit Lesion Plaque Morphology in Patients with ST-Segment-Elevation Myocardial Infarction: Results From the COMPLETE Trial Optical Coherence Tomography Substudys. Circ. Cardiovasc. Interv. 2020, 13, e008768. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B.; Lappé, D.L.; Lima, J.A.C.; Rosen, B.D.; May, H.T.; Knight, S.; Bluemke, D.A.; Towner, S.R.; Le, V.; Bair, T.L.; et al. Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High-Risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial. JAMA 2014, 312, 2234–2243. [Google Scholar] [CrossRef]
- Konduracka, E.; Cieslik, G.; Malecki, M.T.; Gajos, G.; Siniarski, A.; Malinowski, K.P.; Kostkiewicz, M.; Mastalerz, L.; Nessler, J.; Piwowarska, W. Obstructive and Nonobstructive Coronary Artery Disease in Long-Lasting Type 1 Diabetes: A 7-Year Prospective Cohort Study. Pol. Arch. Intern. Med. 2019, 129, 97–105. [Google Scholar] [CrossRef]
- Svanteson, M.; Holte, K.B.; Haig, Y.; Kløw, N.E.; Berg, T.J. Coronary Plaque Characteristics and Epicardial Fat Tissue in Long Term Survivors of Type 1 Diabetes Identified by Coronary Computed Tomography Angiography. Cardiovasc. Diabetol. 2019, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Holte, K.B.; Svanteson, M.; Hanssen, K.F.; Haig, Y.; Solheim, S.; Berg, T.J. Undiagnosed Coronary Artery Disease in Long-Term Type 1 Diabetes. The Dialong Study. J. Diabetes Complicat. 2019, 33, 383–389. [Google Scholar] [CrossRef]
- Madaj, P.M.; Budoff, M.J.; Li, D.; Tayek, J.A.; Karlsberg, R.P.; Karpman, H.L. Identification of Noncalcified Plaque in Young Persons with Diabetes: An Opportunity for Early Primary Prevention of Coronary Artery Disease Identified with Low-Dose Coronary Computed Tomographic Angiography. Acad. Radiol. 2012, 19, 889–893. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Cheng, P.; Liao, H.; Jiang, C.; Li, Y.; Liu, S.; Xu, X. Lower Limb Arterial Calcification and Its Clinical Relevance with Peripheral Arterial Disease. Front. Cardiovasc. Med. 2023, 10, 1271100. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Ato, D. Pitfalls in the Ankle-Brachial Index and Brachial-Ankle Pulse Wave Velocity. Vasc. Health Risk Manag. 2018, 14, 41–62. [Google Scholar] [CrossRef]
- Giménez-Pérez, G.; Viñals, C.; Mata-Cases, M.; Vlacho, B.; Real, J.; Franch-Nadal, J.; Ortega, E.; Mauricio, D. Epidemiology of the First-Ever Cardiovascular Event in People with Type 1 Diabetes: A Retrospective Cohort Population-Based Study in Catalonia. Cardiovasc. Diabetol. 2023, 22, 179. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gibbons, C.H.; Giurini, J.M.; Hilliard, M.E.; Johnson, E.L.; et al. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S231–S243. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Evans, C.V.; Redmond, N.; Lin, J.S. Screening for Peripheral Artery Disease Using the Ankle-Brachial Index: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Chuter, V.H.; Searle, A.; Barwick, A.; Golledge, J.; Leigh, L.; Oldmeadow, C.; Peterson, B.; Tehan, P.; Twigg, S.M. Estimating the Diagnostic Accuracy of the Ankle-Brachial Pressure Index for Detecting Peripheral Arterial Disease in People with Diabetes: A Systematic Review and Meta-Analysis. Diabetes Med. 2021, 38, e14379. [Google Scholar] [CrossRef]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Ankle-Brachial Index Measured by Oscillometry Is Predictive for Cardiovascular Disease and Premature Death in the Japanese Population: An Individual Participant Data Meta-Analysis. Atherosclerosis 2018, 275, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Man, C.; Zhang, H.; Fan, Y. High Ankle-Brachial Index and Risk of Cardiovascular or All-Cause Mortality: A Meta-Analysis. Atherosclerosis 2019, 282, 29–36. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Nie, F.; Hu, X. Prognostic Value of Abnormal Ankle-Brachial Index in Patients with Coronary Artery Disease: A Meta-Analysis. Angiology 2020, 71, 491–497. [Google Scholar] [CrossRef]
- Nie, F.; He, J.; Cao, H.; Hu, X. Predictive Value of Abnormal Ankle-Brachial Index in Patients with Diabetes: A Meta-Analysis. Diabetes Res. Clin. Pract. 2021, 174, 108723. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, H.; Liu, F.; Shen, J.; Li, L.; Zhao, J.; Zhao, J.; Jia, W. High Ankle-Brachial Index Indicates Cardiovascular and Peripheral Arterial Disease in Patients with Type 2 Diabetes. Angiology 2015, 66, 918–924. [Google Scholar] [CrossRef]
- Nattero-Chávez, L.; Redondo López, S.; Alonso Díaz, S.; Garnica Ureña, M.; Fernández-Durán, E.; Escobar-Morreale, H.F.; Luque-Ramírez, M. The Peripheral Atherosclerotic Profile in Patients with Type 1 Diabetes Warrants a Thorough Vascular Assessment of Asymptomatic Patients. Diabetes Metab. Res. Rev. 2019, 35, e3088. [Google Scholar] [CrossRef]
- Ix, J.H.; Miller, R.G.; Criqui, M.H.; Orchard, T.J. Test Characteristics of the Ankle-Brachial Index and Ankle-Brachial Difference for Medial Arterial Calcification on X-Ray in Type 1 Diabetes. J. Vasc. Surg. 2012, 56, 721–727. [Google Scholar] [CrossRef]
- Hajhosseiny, R.; Bahaei, T.S.; Prieto, C.; Botnar, R.M. Molecular and Nonmolecular Magnetic Resonance Coronary and Carotid Imaging. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 569–582. [Google Scholar] [CrossRef]
- Chang, S.A.; Kim, R.J. The Use of Cardiac Magnetic Resonance in Patients with Suspected Coronary Artery Disease: A Clinical Practice Perspective. J. Cardiovasc. Ultrasound 2016, 24, 96. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, S.; Findeisen, H.M.; Schoenberg, S.O.; Kramer, H.; Stark, R.; Clevert, D.A.; Reiser, M.F.; Parhofer, K.G. Systemic Cardiovascular Complications in Patients with Long-Standing Diabetes Mellitus: Comprehensive Assessment with Whole-Body Magnetic Resonance Imaging/Magnetic Resonance Angiography. Investig. Radiol. 2009, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Astrup, A.S.; Stuber, M.; Tarnow, L.; Falk, E.; Botnar, R.M.; Simonsen, C.; Pietraszek, L.; Hansen, P.R.; Manning, W.J.; et al. Subclinical Coronary and Aortic Atherosclerosis Detected by Magnetic Resonance Imaging in Type 1 Diabetes with and without Diabetic Nephropathy. Circulation 2007, 115, 228–235. [Google Scholar] [CrossRef] [PubMed]
- González-Clemente, J.M.; Cano, A.; Albert, L.; Giménez-palop, O.; Romero, A.; Berlanga, E.; Vendrell, J.; Llauradó, G. Arterial Stiffness in Type 1 Diabetes: The Case for the Arterial Wall Itself as a Target Organ. J. Clin. Med. 2021, 10, 3616. [Google Scholar] [CrossRef] [PubMed]
- Bender, Y.Y.; Pfeifer, A.; Ebersberger, H.U.; Diederichs, G.; Hoppe, P.; Hamm, B.; Botnar, R.M.; Makowski, M.R. Molecular Cardiovascular Magnetic Resonance: Current Status and Future Prospects. Curr. Cardiol. Rep. 2016, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Senders, M.L.; Calcagno, C.; Tawakol, A.; Nahrendorf, M.; Mulder, W.J.M.; Fayad, Z.A. PET/MR Imaging of Inflammation in Atherosclerosis. Nat. Biomed. Eng. 2023, 7, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Zobel, E.H.; Winther, S.A.; Hasbak, P.; Von Scholten, B.J.; Holmvang, L.; Kjaer, A.; Rossing, P.; Hansen, T.W. Myocardial Flow Reserve Assessed by Cardiac 82Rb Positron Emission Tomography/Computed Tomography Is Associated with Albumin Excretion in Patients with Type 1 Diabetes. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Pavon, A.G.; Angeli, F.; Tuttolomondo, D.; Belmonte, M.; Armillotta, M.; Sansonetti, A.; Foà, A.; Paolisso, P.; Baggiano, A.; et al. The Role of Non-Invasive Multimodality Imaging in Chronic Coronary Syndrome: Anatomical and Functional Pathways. Diagnostics 2023, 13, 2083. [Google Scholar] [CrossRef]
- Morrone, D.; Gentile, F.; Aimo, A.; Cameli, M.; Barison, A.; Picoi, M.E.; Guglielmo, M.; Villano, A.; DeVita, A.; Mandoli, G.E.; et al. Perspectives in Noninvasive Imaging for Chronic Coronary Syndromes. Int. J. Cardiol. 2022, 365, 19–29. [Google Scholar] [CrossRef]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; De Vega, V.M.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort the PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef]


| Imaging Modality | Strengths | Limitations |
|---|---|---|
| Carotid US | Identifies atherosclerosis in early stages Enables the differentiation of some plaque characteristics (better characterisation if CEUS or 3DVUS is used) Low cost No radiation exposure | Operator-dependent Lack of methodological standardisation of IMT measurements |
| CAC | Reproducible and standardised Strong CVD predictor Low cost | Radiation exposure (low) Does not identify plaque in early stages (only detects calcified plaques) Doubtful usefulness for monitoring treatment (promotion of plaque calcification with statin use) |
| CCTA | Reproducible and standardised Detects obstructive coronary lesions and degree of stenosis (direct detection of CAD) Plaque characterisation | Expensive Radiation exposure Iodinated contrast exposure (allergy, nephrotoxicity) Imaging is limited if arrhythmias and/or obesity Blooming artefact in calcified lesions |
| ABI | Standardised Easy-to-interpret results Inexpensive No radiation exposure Quick to perform | Does not visualise atherosclerosis Primarily picks up hemodynamically significant lesions and therefore may miss subclinical disease Limited interpretation in certain patient populations (high calcification burden, haemodialysis) |
| MR | Excellent soft tissue resolution for plaque characterisation Multiparametric (morphology, functionality) No radiation exposure | Motion artefacts (mainly limited to large-calibre vessels) Expensive Time consuming |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serés-Noriega, T.; Perea, V.; Amor, A.J. Screening for Subclinical Atherosclerosis and the Prediction of Cardiovascular Events in People with Type 1 Diabetes. J. Clin. Med. 2024, 13, 1097. https://doi.org/10.3390/jcm13041097
Serés-Noriega T, Perea V, Amor AJ. Screening for Subclinical Atherosclerosis and the Prediction of Cardiovascular Events in People with Type 1 Diabetes. Journal of Clinical Medicine. 2024; 13(4):1097. https://doi.org/10.3390/jcm13041097
Chicago/Turabian StyleSerés-Noriega, Tonet, Verónica Perea, and Antonio J. Amor. 2024. "Screening for Subclinical Atherosclerosis and the Prediction of Cardiovascular Events in People with Type 1 Diabetes" Journal of Clinical Medicine 13, no. 4: 1097. https://doi.org/10.3390/jcm13041097