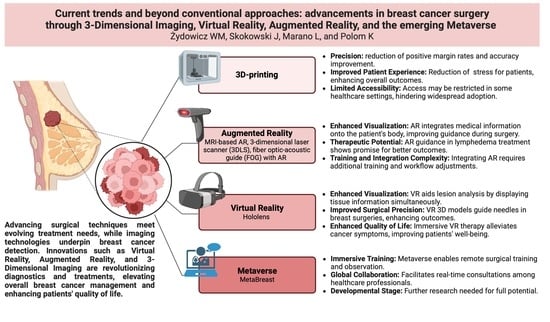
Current Trends and Beyond Conventional Approaches: Advancements in Breast Cancer Surgery through Three-Dimensional Imaging, Virtual Reality, Augmented Reality, and the Emerging Metaverse
Abstract
:1. Introduction
2. Materials and Methods
3. Technologies
3.1. 3D Printing
3.1.1. Materials
3.1.2. Advantages and Disadvantages
3.2. Augmented Reality
Techniques—Advantages and Disadvantages
3.3. Virtual Reality
Techniques—Advantages and Disadvantages
3.4. Metaverse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lu, G.; Qin, B.; Fei, B. Ultrasound imaging technologies for breast cancer detection and management—A review. Ultrasound Med. Biol. 2018, 44, 37. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Matsen, C.B.; Neumayer, L.A. Breast Cancer: A Review for the General Surgeon. JAMA Surg. 2013, 148, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Moroni, S.; Casettari, L.; Lamprou, D.A. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.; Kerr, G.R. The Curability of Breast Cancer. Br. Med. J. 1976, 2, 781–783. [Google Scholar] [CrossRef]
- Costa, N.; Ferreira, L.; de Araújo, A.R.V.F.; Oliveira, B.; Torres, H.R.; Morais, P.; Alves, V.; Vilaça, J.L. Augmented Reality-Assisted Ultrasound Breast Biopsy. Sensors 2023, 23, 1838. [Google Scholar] [CrossRef]
- Kukla, P.; Maciejewska, K.; Strojna, I.; Zapał, M.; Zwierzchowski, G.; Bąk, B. Extended Reality in Diagnostic Imaging-A Literature Review. Tomography 2023, 9, 1071–1082. [Google Scholar] [CrossRef]
- Taghian, A.; Abo-Zahhad, M.; Sayed, M.S.; Abd El-Malek, A.H. Virtual and Augmented Reality in Biomedical Engineering. Biomed Eng. Online 2023, 22, 76. [Google Scholar] [CrossRef]
- Costa, J.N.; Gomes-Fonseca, J.; Valente, S.; Ferreira, L.; Oliveira, B.; Torres, H.R.; Morais, P.; Alves, V.; Vilaca, J.L. Ultrasound Training Simulator Using Augmented Reality Glasses: An Accuracy and Precision Assessment Study. In Proceedings of the 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Glasgow, UK, 11–15 July 2022; Volume 2022, pp. 4461–4464. [Google Scholar] [CrossRef]
- Nguyen, T.; Plishker, W.; Matisoff, A.; Sharma, K.; Shekhar, R. HoloUS: Augmented Reality Visualization of Live Ultrasound Images Using HoloLens for Ultrasound-Guided Procedures. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 385–391. [Google Scholar] [CrossRef]
- Gouveia, P.F.; Luna, R.; Fontes, F.; Pinto, D.; Mavioso, C.; Anacleto, J.; Timóteo, R.; Santinha, J.; Marques, T.; Cardoso, F.; et al. Augmented Reality in Breast Surgery Education. Breast Care 2023, 18, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Tetzlaff, J.; Moher, D. The Art and Science of Knowledge Synthesis. J. Clin. Epidemiol. 2011, 64, 11–20. [Google Scholar] [CrossRef] [PubMed]
- What Is Medical 3D Printing and How Does It Work?|Synopsys. Available online: https://www.synopsys.com/glossary/what-is-medical-3d-printing.html (accessed on 27 March 2023).
- Galstyan, A.; Bunker, M.J.; Lobo, F.; Sims, R.; Inziello, J.; Stubbs, J.; Mukhtar, R.; Kelil, T. Applications of 3D Printing in Breast Cancer Management. 3D Print. Med. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, L.; Wake, N.; Ryan, J.; Althobaity, W.; Gupta, A.; Arribas, E.; Santiago, L.; Ballard, D.H.; Wang, K.C.; Weadock, W.; et al. Radiological Society of North America (RSNA) 3D Printing Special Interest Group (SIG): Guidelines for Medical 3D Printing and Appropriateness for Clinical Scenarios. 3D Print Med. 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.J.; Krishnaswamy, V.; Paulsen, K.D.; Rooney, T.B.; Wells, W.A.; Rizzo, E.; Angeles, C.V.; Marotti, J.D.; Zuurbier, R.A.; Black, C.C. A Patient-Specific 3D-Printed Form Accurately Transfers Supine MRI-Derived Tumor Localization Information to Guide Breast-Conserving Surgery. Ann. Surg. Oncol. 2017, 24, 2950. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, M.; Nagashima, T.; Sangai, T.; Nakamura, R.; Fujimoto, H.; Arai, M.; Kazama, T.; Hashimoto, H.; Nakatani, Y.; Miyazaki, M. Breast-Conserving Surgery Using Projection and Reproduction Techniques of Surgical-Position Breast MRI in Patients with Ductal Carcinoma in Situ of the Breast. J. Am. Coll. Surg. 2008, 207, 62–68. [Google Scholar] [CrossRef]
- Pallone, M.J.; Poplack, S.P.; Avutu, H.B.R.; Paulsen, K.D.; Barth, R.J. Supine Breast MRI and 3D Optical Scanning: A Novel Approach to Improve Tumor Localization for Breast Conserving Surgery. Ann. Surg. Oncol. 2014, 21, 2203–2208. [Google Scholar] [CrossRef]
- Krekel, N.M.A.; Haloua, M.H.; Lopes Cardozo, A.M.F.; de Wit, R.H.; Bosch, A.M.; de Widt-Levert, L.M.; Muller, S.; van der Veen, H.; Bergers, E.; de Lange de Klerk, E.S.M.; et al. Intraoperative Ultrasound Guidance for Palpable Breast Cancer Excision (COBALT Trial): A Multicentre, Randomised Controlled Trial. Lancet Oncol. 2013, 14, 48–54. [Google Scholar] [CrossRef]
- Moore, M.M.; Whitney, L.A.; Cerilli, L.; Imbrie, J.Z.; Bunch, M.; Simpson, V.B.; Hanks, J.B. Intraoperative Ultrasound Is Associated with Clear Lumpectomy Margins for Palpable Infiltrating Ductal Breast Cancer. Ann. Surg. 2001, 233, 761–768. [Google Scholar] [CrossRef]
- Tomikawa, M.; Hong, J.; Shiotani, S.; Tokunaga, E.; Konishi, K.; Ieiri, S.; Tanoue, K.; Akahoshi, T.; Maehara, Y.; Hashizume, M. Real-Time 3-Dimensional Virtual Reality Navigation System with Open MRI for Breast-Conserving Surgery. J. Am. Coll. Surg. 2010, 210, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic Accuracy of Mammography, Clinical Examination, US, and MR Imaging in Preoperative Assessment of Breast Cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Menell, J.H.; Morris, E.A.; Dershaw, D.D.; Abramson, A.F.; Brogi, E.; Liberman, L. Determination of the Presence and Extent of Pure Ductal Carcinoma in Situ by Mammography and Magnetic Resonance Imaging. Breast J. 2005, 11, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Kinkel, K.; Esserman, L.J.; Lu, Y.; Weidner, N.; Hylton, N.M. Magnetic Resonance Imaging in Patients Diagnosed with Ductal Carcinoma-in-Situ: Value in the Diagnosis of Residual Disease, Occult Invasion, and Multicentricity. Ann. Surg. Oncol. 2003, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- What Is Augmented Reality (AR)|Microsoft Dynamics 365. Available online: https://dynamics.microsoft.com/en-us/mixed-reality/guides/what-is-augmented-reality-ar/ (accessed on 26 February 2023).
- Rancati, A.; Angrigiani, C.; Nava, M.B.; Catanuto, G.; Rocco, N.; Ventrice, F.; Dorr, J. Augmented Reality for Breast Imaging. Minerva Chir. 2018, 73, 341–344. [Google Scholar] [CrossRef] [PubMed]
- What Are the Applications of Augmented Reality (AR) in Manufacturing?—PreScouter—Custom Intelligence from a Global Network of Experts. Available online: https://www.prescouter.com/2019/05/what-are-the-applications-of-augmented-reality-ar-in-manufacturing/ (accessed on 27 March 2023).
- Kim, K.; Parky, Y.; Wooz, W. Digilog Miniature: Real-Time, Immersive, and Interactive AR on Miniatures. In Proceedings of the VRCAI 2010, ACM SIGGRAPH Conference on Virtual-Reality Continuum and Its Application to Industry, Seoul, Republic of Korea, 12–13 December 2010; pp. 161–168. [Google Scholar] [CrossRef]
- Games Are Not Everything: How VR/AR Technology Changes the Face of Education—ITCorner. Available online: https://itcorner.org.pl/blog/games-are-not-everything-how-vr-ar-technology-changes-the-face-of-education/ (accessed on 27 February 2023).
- Augmented Reality—Wikipedia. Available online: https://en.wikipedia.org/wiki/Augmented_reality (accessed on 27 March 2023).
- Besl, P.J.; McKay, N.D. A Method for Registration of 3-D Shapes. IEEE Trans. Pattern Anal. Mach. Intell. 1992, 14, 239–256. [Google Scholar] [CrossRef]
- Khang, S.; Park, T.; Lee, J.; Kim, K.W.; Song, H.; Lee, J. Computer-Aided Breast Surgery Framework Using a Markerless Augmented Reality Method. Diagnostics 2022, 12, 3123. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, K.E.; Zuckerman, S.P.; Weinstein, S.P.; Tobey, J.; Birnbaum, J.A.; McDonald, E.S.; Conant, E.F. Breast MRI: False-Negative Results and Missed Opportunities. Radiographics 2021, 41, 645–664. [Google Scholar] [CrossRef]
- Duraes, M.; Crochet, P.; Pagès, E.; Grauby, E.; Lasch, L.; Rebel, L.; Van Meer, F.; Rathat, G. Surgery of Nonpalpable Breast Cancer: First Step to a Virtual per-Operative Localization? First Step to Virtual Breast Cancer Localization. Breast J. 2019, 25, 874–879. [Google Scholar] [CrossRef]
- Vavourakis, V.; Eiben, B.; Hipwell, J.H.; Williams, N.R.; Keshtgar, M.; Hawkes, D.J. Multiscale Mechano-Biological Finite Element Modelling of Oncoplastic Breast Surgery—Numerical Study towards Surgical Planning and Cosmetic Outcome Prediction. PLoS ONE 2016, 11, e0159766. [Google Scholar] [CrossRef]
- Reece, G.P.; Merchant, F.; Andon, J.; Khatam, H.; Ravi-Chandar, K.; Weston, J.; Fingeret, M.C.; Lane, C.; Duncan, K.; Markey, M.K. 3D Surface Imaging of the Human Female Torso in Upright to Supine Positions. Med. Eng. Phys. 2015, 37, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, P.F.; Costa, J.; Morgado, P.; Kates, R.; Pinto, D.; Mavioso, C.; Anacleto, J.; Martinho, M.; Lopes, D.S.; Ferreira, A.R.; et al. Breast Cancer Surgery with Augmented Reality. Breast 2021, 56, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Xia, Y.; Li, R.; Liu, K.; Mai, J.; Medley, J.A.; Obeng-Gyasi, S.; Han, L.K.; Wang, P.; Cheng, J.X. A Fiber Optoacoustic Guide with Augmented Reality for Precision Breast-Conserving Surgery. Light Sci. Appl. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.; Mushawah, F.A.; Appleton, C.M.; Cyr, A.E.; Gillanders, W.E.; Aft, R.L.; Eberlein, T.J.; Gao, F.; Margenthaler, J.A. Positive Margins Rates Following Breast-Conserving Surgery for Stage I–III Breast Cancer: Palpable versus Non-Palpable Tumors. J. Surg. Res. 2012, 177, 109. [Google Scholar] [CrossRef] [PubMed]
- Valdes, E.K.; Boolbol, S.K.; Cohen, J.M.; Feldman, S.M. Intra-Operative Touch Preparation Cytology; Does It Have a Role in Re-Excision Lumpectomy? Ann. Surg. Oncol. 2007, 14, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.J.; Hill, A.D.K.; Mc Dermott, E.W.; O’Doherty, A.; O’Higgins, N.J.; Quinn, C.M. Intraoperative Margin Assessment and Re-Excision Rate in Breast Conserving Surgery. Eur. J. Surg. Oncol. 2004, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Huston, T.L.; Pigalarga, R.; Osborne, M.P.; Tousimis, E. The Influence of Additional Surgical Margins on the Total Specimen Volume Excised and the Reoperative Rate after Breast-Conserving Surgery. Am. J. Surg. 2006, 192, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duric, N.; Littrup, P.; Huang, L. In Vivo Breast Sound-Speed Imaging with Ultrasound Tomography. Ultrasound Med. Biol. 2009, 35, 1615–1628. [Google Scholar] [CrossRef]
- Michelotti, A.; Invernizzi, M.; Lopez, G.; Lorenzini, D.; Nesa, F.; De Sire, A.; Fusco, N. Tackling the Diversity of Breast Cancer Related Lymphedema: Perspectives on Diagnosis, Risk Assessment, and Clinical Management. Breast 2019, 44, 15–23. [Google Scholar] [CrossRef]
- Noguchi, M. Axillary Reverse Mapping for Breast Cancer. Breast Cancer Res. Treat. 2010, 119, 529–535. [Google Scholar] [CrossRef]
- Dean, L.T.; Moss, S.L.; Ransome, Y.; Frasso-Jaramillo, L.; Zhang, Y.; Visvanathan, K.; Nicholas, L.H.; Schmitz, K.H. “It Still Affects Our Economic Situation”: Long-Term Economic Burden of Breast Cancer and Lymphedema. Support. Care Cancer 2019, 27, 1697–1708. [Google Scholar] [CrossRef]
- Invernizzi, M.; Corti, C.; Lopez, G.; Michelotti, A.; Despini, L.; Gambini, D.; Lorenzini, D.; Guerini-Rocco, E.; Maggi, S.; Noale, M.; et al. Lymphovascular Invasion and Extranodal Tumour Extension Are Risk Indicators of Breast Cancer Related Lymphoedema: An Observational Retrospective Study with Long-Term Follow-up 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. BMC Cancer 2018, 18, 935. [Google Scholar] [CrossRef] [PubMed]
- Hameeteman, M.; Verhulst, A.C.; Vreeken, R.D.; Maal, T.J.J.; Ulrich, D.J.O. 3D Stereophotogrammetry in Upper-Extremity Lymphedema: An Accurate Diagnostic Method. J. Plast Reconstr. Aesthet. Surg. 2016, 69, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Cau, N.; Galli, M.; Cimolin, V.; Aranci, M.; Caraceni, A.; Balzarini, A. Comparative Study between Circumferential Method and Laser Scanner 3D Method for the Evaluation of Arm Volume in Healthy Subjects. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Runza, L.; De Sire, A.; Lippi, L.; Blundo, C.; Gambini, D.; Boldorini, R.; Ferrero, S.; Fusco, N. Integrating Augmented Reality Tools in Breast Cancer Related Lymphedema Prognostication and Diagnosis. J. Vis. Exp. 2020, 156, 60093. [Google Scholar] [CrossRef]
- Tinterri, C.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Jacobs, F.; Zambelli, A.; Trimboli, R.M.; Bernardi, D.; Vinci, V.; Gentile, D. Loco-Regional Treatment of the Primary Tumor in De Novo Metastatic Breast Cancer Patients Undergoing Front-Line Chemotherapy. Cancers 2022, 14, 6237. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Anghelone, C.A.P.; Gentile, D.; La Raja, C.; Bottini, A.; Tinterri, C. Metastases from Occult Breast Cancer: A Case Report of Carcinoma of Unknown Primary Syndrome. Case Rep. Oncol. 2020, 13, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Brebant, V.; Heine, N.; Lamby, P.; Heidekrueger, P.I.; Forte, A.J.; Prantl, L.; Aung, T. Augmented Reality of Indocyanine Green Fluorescence in Simplified Lymphovenous Anastomosis in Lymphatic Surgery. Clin. Hemorheol. Microcirc. 2019, 73, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Muralidharan, A.; Cuasay, J.; Pruthi, S.; Kesavadas, T. An Augmented Reality Application for Clinical Breast Examination Training. In Proceedings of the 2021 4th IEEE International Conference on Artificial Intelligence and Virtual Reality, AIVR 2021, Taichung, Taiwan, 5–17 November 2021; pp. 224–227. [Google Scholar] [CrossRef]
- Virtual Reality|Definition, Development, Technology, Examples, & Facts|Britannica. Available online: https://www.britannica.com/technology/virtual-reality (accessed on 26 February 2023).
- Hellwig, G.; Brix, G.; Griebel, J.; Lucht, R.; Delorme, S.; Siebert, M.; Englmeier, K.H. Dynamic MR Mammography: Three-Dimensional Real-Time Visualization of Contrast Enhancement in Virtual Reality. Acad. Radiol. 2002, 9, 1255–1263. [Google Scholar] [CrossRef]
- Navigated Breast Cancer Surgery Using Open-Source Software (PLUS, 3D). Download Scientific Diagram. Available online: https://www.researchgate.net/figure/Navigated-breast-cancer-surgery-using-open-source-software-PLUS-3D-Slicer-and_fig3_304107611 (accessed on 4 April 2023).
- Image-Detected Breast Cancer: State of the Art Diagnosis and Treatment. J. Am. Coll. Surg. 2001, 193, 297–302. [CrossRef] [PubMed]
- Eby, P.R.; Lehman, C.D. Magnetic Resonance Imaging--Guided Breast Interventions. Top. Magn. Reson. Imaging 2008, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.E.; Daily, S.; Tometich, D.; Matthias, M.S.; Outcalt, S.D.; Hirsh, A.; Johns, S.A.; Rand, K.; Schneider, B.; Mina, L.; et al. Factors Underlying Metastatic Breast Cancer Patients’ Perceptions of Symptom Importance: A Qualitative Analysis. Eur. J. Cancer Care 2018, 27, e12540. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, A.; Paty, J.; Rydén, A. Talking About Breast Cancer: Which Symptoms and Treatment Side Effects Are Important to Patients with Advanced Disease? Patient 2017, 10, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.; von Moos, R.; Walker, M.S.; Wang, Y.; Gao, J.; Chavez-MacGregor, M.; Liede, A.; Arellano, J.; Balakumaran, A.; Qian, Y. Burden of Symptoms Associated with Development of Metastatic Bone Disease in Patients with Breast Cancer. Support. Care Cancer 2016, 24, 3557–3565. [Google Scholar] [CrossRef]
- Park, E.M.; Gelber, S.; Rosenberg, S.M.; Seah, D.S.E.; Schapira, L.; Come, S.E.; Partridge, A.H. Anxiety and Depression in Young Women With Metastatic Breast Cancer: A Cross-Sectional Study. Psychosomatics 2018, 59, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Saggino, A.; Agostini, F.; Paoloni, M.; Bernetti, A.; Mangone, M.; Santilli, V.; Saggini, R.; Tommasi, M. The Influence of Rehabilitation on Quality of Life in Breast Cancer Survivors: A Clinical Study. Int. J. Environ. Res. Public Health 2021, 18, 8585. [Google Scholar] [CrossRef]
- Teo, I.; Krishnan, A.; Lee, G.L. Psychosocial Interventions for Advanced Cancer Patients: A Systematic Review. Psychooncology 2019, 28, 1394–1407. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Cavadino, A.; Chin, S.; Little, Z.; Akroyd, A.; Tennant, G.; Dobson, R.; Broom, R.; Gautier, A. The Benefits and Acceptability of Virtual Reality Interventions for Women with Metastatic Breast Cancer in Their Homes; a Pilot Randomised Trial. BMC Cancer 2022, 22, 360. [Google Scholar] [CrossRef]
- Ventura, S.; Baños, R.M.; Botella, C.; Ventura, S.; Baños, R.M.; Botella, C. Virtual and Augmented Reality: New Frontiers for Clinical Psychology. In State of the Art Virtual Reality and Augmented Reality Knowhow; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Patterson, D.R.; Seibel, E.; Soltani, M.; Jewett-Leahy, L.; Sharar, S.R. Virtual Reality Pain Control during Burn Wound Debridement in the Hydrotank. Clin. J. Pain 2008, 24, 299–304. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual Reality in Health System: Beyond Entertainment. A Mini-Review on the Efficacy of VR During Cancer Treatment. J. Cell. Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Aziz, N.; Fahey, J.L. Fatigue and Proinflammatory Cytokine Activity in Breast Cancer Survivors. Psychosom. Med. 2002, 64, 604–611. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Aziz, N.; Fahey, J.L.; Cole, S.W. T-Cell Homeostasis in Breast Cancer Survivors with Persistent Fatigue. J. Natl. Cancer Inst. 2003, 95, 1165–1168. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Moore, T.; Choo, J. The Impact of Virtual Reality on Chronic Pain. PLoS ONE 2016, 11, e0167523. [Google Scholar] [CrossRef] [PubMed]
- Ganry, L.; Hersant, B.; Sidahmed-Mezi, M.; Dhonneur, G.; Meningaud, J.P. Using Virtual Reality to Control Preoperative Anxiety in Ambulatory Surgery Patients: A Pilot Study in Maxillofacial and Plastic Surgery. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 257–261. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Patterson, D.R.; Carrougher, G.J.; Sharar, S.R. Effectiveness of Virtual Reality-Based Pain Control with Multiple Treatments. Clin. J. Pain 2001, 17, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Prince-Paul, M.; Allen, M.J.; Silverman, P.; Talaba, D. Virtual Reality as a Distraction Intervention for Women Receiving Chemotherapy. Oncol. Nurs. Forum 2004, 31, 81–88. [Google Scholar] [CrossRef]
- Gerçeker, G.Ö.; Bektaş, M.; Aydınok, Y.; Ören, H.; Ellidokuz, H.; Olgun, N. The Effect of Virtual Reality on Pain, Fear, and Anxiety during Access of a Port with Huber Needle in Pediatric Hematology-Oncology Patients: Randomized Controlled Trial. Eur. J. Oncol. Nurs. 2021, 50, 101886. [Google Scholar] [CrossRef]
- Baños, R.M.; Espinoza, M.; García-Palacios, A.; Cervera, J.M.; Esquerdo, G.; Barrajón, E.; Botella, C. A Positive Psychological Intervention Using Virtual Reality for Patients with Advanced Cancer in a Hospital Setting: A Pilot Study to Assess Feasibility. Support. Care Cancer 2013, 21, 263–270. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, N.; Zhang, H.; Sun, X.; Wang, Y.; Zhang, Y. Effectiveness of Virtual Reality in Symptom Management of Cancer Patients: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2023, 65, e467–e482. [Google Scholar] [CrossRef]
- McWilliam, A.; Scarfe, P. The Metaverse and Oncology. Clin. Oncol. 2023, 35, 12–14. [Google Scholar] [CrossRef]
- Kye, B.; Han, N.; Kim, E.; Park, Y.; Jo, S. Educational Applications of Metaverse: Possibilities and Limitations. J. Educ. Eval. Health Prof. 2021, 18, 32. [Google Scholar] [CrossRef]
- Digital Health in the Metaverse: Three Legal Considerations|Healthcare Law Blog. Available online: https://www.sheppardhealthlaw.com/2022/05/articles/digital-health/digital-health-metaverse-legal/?utm_source=Mondaq&utm_medium=syndication&utm_campaign=LinkedIn-integration (accessed on 26 February 2023).
- Yang, D.; Zhou, J.; Chen, R.; Song, Y.; Song, Z.; Zhang, X.; Wang, Q.; Wang, K.; Zhou, C.; Sun, J.; et al. Expert Consensus on the Metaverse in Medicine. Clin. eHealth 2022, 5, 1–9. [Google Scholar] [CrossRef]
- Home—Roblox. Available online: https://corp.roblox.com/ (accessed on 1 March 2023).
- Epic Games Store|Pobierz i Graj w Gry Na PC, Mody, DLC i Nie Tylko—Epic Games. Available online: https://store.epicgames.com/pl/ (accessed on 1 March 2023).
- Metaverse May Be $800 Billion Market, Next Tech Platform|Insights|Bloomberg Professional Services. Available online: https://www.bloomberg.com/professional/blog/metaverse-may-be-800-billion-market-next-tech-platform/ (accessed on 26 February 2023).
- Zeng, Y.; Zeng, L.; Zhang, C.; Cheng, A.S.K. The Metaverse in Cancer Care: Applications and Challenges. Asia Pac. J. Oncol. Nurs. 2022, 9, 100111. [Google Scholar] [CrossRef]
- Koo, H. Training in Lung Cancer Surgery through the Metaverse, Including Extended Reality, in the Smart Operating Room of Seoul National University Bundang Hospital, Korea. J. Educ. Eval. Health Prof. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- [Smart! Economy] Shall We Go before Corona… Daily Life on the “Metaverse”|JTBC News. Available online: https://news.jtbc.co.kr/article/article.aspx?news_id=NB12014875 (accessed on 1 March 2023).
- Chand, M. LinkedIn: #vr #education #healthcare #metaverse #surgery. Available online: https://www.linkedin.com/posts/manish-chand-md-mba-phd-48490a71_vr-education-healthcare-activity-7021047823194288128-_iGP?originalSubdomain=nl (accessed on 4 April 2023).
- What Is Metaverse? By Mahesh Chand—YouTube. Available online: https://www.youtube.com/watch?v=qJS3ZRKoGmw&feature=youtu.be&ab_channel=C%23Corner (accessed on 4 April 2023).
- The Future of Breast Cancer Surgery Is Coming—with the Help of Funding from the Portuguese PRR|Champalimaud Foundation. Available online: https://fchampalimaud.org/news/future-of-breast-cancer-surgery-is-coming (accessed on 17 May 2023).
- BodySCULPT Brings Oculus Rift 3D Imaging and Virtual Reality to Breast Surgery and Body Sculpting. Available online: https://www.prnewswire.com/news-releases/bodysculpt-brings-oculus-rift-3d-imaging-and-virtual-reality-to-breast-surgery-and-body-sculpting-300189394.html (accessed on 17 May 2023).
- Holographic Augmented Reality in Deep Inferior Epigastric Perforator Flap Breast Reconstruction—University of Twente Student Theses. Available online: http://essay.utwente.nl/80356/ (accessed on 28 November 2023).
- “HoloLens in Breast Reconstruction: What Is the Future?”: Plastic and Reconstructive Surgery. Available online: https://journals.lww.com/plasreconsurg/Abstract/9900/_HoloLens_in_breast_reconstruction__what_is_the.1471.aspx (accessed on 17 May 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żydowicz, W.M.; Skokowski, J.; Marano, L.; Polom, K. Current Trends and Beyond Conventional Approaches: Advancements in Breast Cancer Surgery through Three-Dimensional Imaging, Virtual Reality, Augmented Reality, and the Emerging Metaverse. J. Clin. Med. 2024, 13, 915. https://doi.org/10.3390/jcm13030915
Żydowicz WM, Skokowski J, Marano L, Polom K. Current Trends and Beyond Conventional Approaches: Advancements in Breast Cancer Surgery through Three-Dimensional Imaging, Virtual Reality, Augmented Reality, and the Emerging Metaverse. Journal of Clinical Medicine. 2024; 13(3):915. https://doi.org/10.3390/jcm13030915
Chicago/Turabian StyleŻydowicz, Weronika Magdalena, Jaroslaw Skokowski, Luigi Marano, and Karol Polom. 2024. "Current Trends and Beyond Conventional Approaches: Advancements in Breast Cancer Surgery through Three-Dimensional Imaging, Virtual Reality, Augmented Reality, and the Emerging Metaverse" Journal of Clinical Medicine 13, no. 3: 915. https://doi.org/10.3390/jcm13030915