Treatment of Refractory Cardiac Arrest by Controlled Reperfusion of the Whole Body: A Multicenter, Prospective Observational Study
Abstract
:1. Introduction
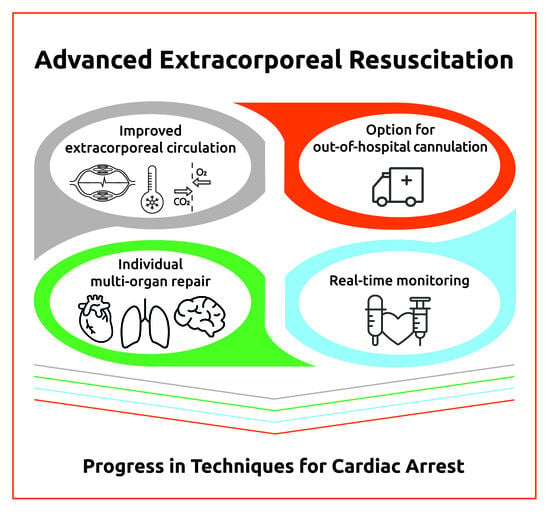
- Extracorporeal circulation: As opposed to regular extracorporeal circuits, CARL contains dual diagonal pumps for pulsatility and high pressure and flow (Supplementary Materials, Figure S1) as well as controlled oxygenation to limit free radical injury. It induces immediate (<20 min) systemic mild hypothermia (33–35 °C).
- Individualized multi-organ repair: Instead of returning the usually hyperoxemic, but otherwise unmodified, blood into the femoral artery, the system adjusts 14 blood parameters (e.g., hypocalcemia, hypermagnesemia, hyperosmolarity, low oxygen, free radical scavengers, etc.) [18] to counteract ischemic/hypoxic and reperfusion/reoxygenation injury to diverse organ systems. These adjustments can be made individually for each patient using the data obtained from the real-time monitoring.
- Comprehensive real-time monitoring: This allows for real-time measurements of hemodynamic (cardiac output, heart rate, and blood pressure), metabolic (blood gases and electrolytes), and temperature parameters and provides personalized treatment by an immediate adjustment to the desired value of each parameter.
- Option for out-of-hospital CARL treatment using suitable mobile devices: This allows pre-hospital cannulation and the early start of CARL in order to reduce the duration of CCPR.
2. Materials and Methods
2.1. Clinical Study, Design, and Statistical Analysis Plan
2.2. Study Setting
2.3. Patients
2.4. Primary and Secondary Endpoints
2.5. Procedure
2.6. Patients with “Non-Survivable” CA
2.7. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Pre-Hospital Resuscitation Characteristics
3.3. Primary Outcome
3.4. Time Intervals and Locations before, during, and after CA and CPR
3.5. Organ Function Assessment
3.6. Clinical Events and Complications
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurie, K.G.; Nemergut, E.C.; Yannopoulos, D.; Sweeney, M. The Physiology of Cardiopulmonary Resuscitation. Anesth. Analg. 2016, 122, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Grunau, B.E.; Elmer, J.; Rittenberger, J.C.; Sawyer, K.N.; Kurz, M.C.; Singer, B.; Proudfoot, A.; Callaway, C.W. Prevalence, natural history, and time-dependent outcomes of a multi-center North American cohort of out-of-hospital cardiac arrest extracorporeal CPR candidates. Resuscitation 2017, 117, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Bougouin, W.; Dumas, F.; Lamhaut, L.; Marijon, E.; Carli, P.; Combes, A.; Pirracchio, R.; Aissaoui, N.; Karam, N.; Deye, N.; et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: A registry study. Eur. Heart J. 2020, 41, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; McNally, B.; Tang, F.; Kellermann, A. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation 2014, 130, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Malta Hansen, C.; Kragholm, K.; Pearson, D.A.; Tyson, C.; Monk, L.; Myers, B.; Nelson, D.; Dupre, M.E.; Fosbøl, E.L.; Jollis, J.G.; et al. Association of Bystander and First-Responder Intervention With Survival After Out-of-Hospital Cardiac Arrest in North Carolina, 2010–2013. JAMA 2015, 314, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wang, C.H.; Chi, N.H.; Huang, S.C.; Chou, H.W.; Chou, N.K.; Chen, Y.S. Effect of interplay between age and low-flow duration on neurologic outcomes of extracorporeal cardiopulmonary resuscitation. Intensive Care Med. 2019, 45, 44–54. [Google Scholar] [CrossRef]
- Schluep, M.; Gravesteijn, B.Y.; Stolker, R.J.; Endeman, H.; Hoeks, S.E. One-year survival after in-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation 2018, 132, 90–100. [Google Scholar] [CrossRef]
- Girotra, S.; Nallamothu, B.K.; Spertus, J.A.; Li, Y.; Krumholz, H.M.; Chan, P.S. Trends in survival after in-hospital cardiac arrest. N. Engl. J. Med. 2012, 367, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Siao, F.Y.; Chiu, C.C.; Chiu, C.W.; Chen, Y.C.; Chen, Y.L.; Hsieh, Y.K.; Lee, C.H.; Wu, C.T.; Chou, C.C.; Yen, H.H. Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: Conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation 2015, 92, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.E.; Chan, P.S.; Tang, F.; Nallamothu, B.K.; Girotra, S.; Perman, S.M.; Bose, S.; Daugherty, S.L.; Bradley, S.M. Long-Term Survival Trends of Medicare Patients after In-Hospital Cardiac Arrest: Insights from Get with the Guidelines-Resuscitation®. Resuscitation 2018, 123, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Graesner, J.T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; Van de Voorde, P.; Madar, J.; Zideman, D.; Mentzelopoulos, S.; et al. European Resuscitation Council Guidelines 2021: Executive summary. Resuscitation 2021, 161, 1–60. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Frisch, A.; Rittenberger, J.C.; Callaway, C.W. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: When should we change to novel therapies? Circulation 2013, 128, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lin, J.W.; Yu, H.Y.; Ko, W.J.; Jerng, J.S.; Chang, W.T.; Chen, W.J.; Huang, S.C.; Chi, N.H.; Wang, C.H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yu, H.Y.; Huang, S.C.; Lin, J.W.; Chi, N.H.; Wang, C.H.; Wang, S.S.; Lin, F.Y.; Ko, W.J. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit. Care Med. 2008, 36, 2529–2535. [Google Scholar] [CrossRef]
- Beyersdorf, F.; Trummer, G.; Benk, C.; Pooth, J.S. Application of cardiac surgery techniques to improve the results of cardiopulmonary resuscitation after cardiac arrest: Controlled automated reperfusion of the whole body. JTCVS Open 2021, 8, 47–52. [Google Scholar] [CrossRef]
- Daniele, S.G.; Trummer, G.; Hossmann, K.A.; Vrselja, Z.; Benk, C.; Gobeske, K.T.; Damjanovic, D.; Andrijevic, D.; Pooth, J.S.; Dellal, D.; et al. Brain vulnerability and viability after ischaemia. Nat. Rev. Neurosci. 2021, 22, 553–572. [Google Scholar] [CrossRef]
- Taunyane, I.C.; Benk, C.; Beyersdorf, F.; Foerster, K.; Cristina Schmitz, H.; Wittmann, K.; Mader, I.; Doostkam, S.; Heilmann, C.; Trummer, G. Preserved brain morphology after controlled automated reperfusion of the whole body following normothermic circulatory arrest time of up to 20 minutes. Eur. J. Cardiothorac. Surg. 2016, 50, 1025–1034. [Google Scholar] [CrossRef]
- Andrijevic, D.; Vrselja, Z.; Lysyy, T.; Zhang, S.; Skarica, M.; Spajic, A.; Dellal, D.; Thorn, S.L.; Duckrow, R.B.; Ma, S.; et al. Cellular recovery after prolonged warm ischaemia of the whole body. Nature 2022, 608, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Vrselja, Z.; Daniele, S.G.; Silbereis, J.; Talpo, F.; Morozov, Y.M.; Sousa, A.M.M.; Tanaka, B.S.; Skarica, M.; Pletikos, M.; Kaur, N.; et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature 2019, 568, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Trummer, G.; Benk, C.; Beyersdorf, F. Controlled automated reperfusion of the whole body after cardiac arrest. J. Thorac. Dis. 2019, 11 (Suppl. S10), S1464–S1470. [Google Scholar] [CrossRef]
- Trummer, G.; Supady, A.; Beyersdorf, F.; Scherer, C.; Wengenmayer, T.; Umhau, M.; Benk, C. Controlled automated reperfusion of the whole body after 120 minutes of Cardiopulmonary resuscitation: First clinical report. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 66. [Google Scholar] [CrossRef]
- Philipp, A.; Pooth, J.S.; Benk, C.; Mueller, T.; Lunz, D. Enabling the control of reperfusion parameters in out-of-hospital cardiac arrest: First applications of the CARL system. Perfusion 2023, 38, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Cummins, R.O.; Chamberlain, D.A.; Abramson, N.S.; Allen, M.; Baskett, P.J.; Becker, L.; Bossaert, L.; Delooz, H.H.; Dick, W.F.; Eisenberg, M.S.; et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: The Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 1991, 84, 960–975. [Google Scholar] [PubMed]
- Perkins, G.D.; Jacobs, I.G.; Nadkarni, V.M.; Berg, R.A.; Bhanji, F.; Biarent, D.; Bossaert, L.L.; Brett, S.J.; Chamberlain, D.; de Caen, A.R.; et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation 2015, 132, 1286–1300. [Google Scholar]
- Kiehl, E.L.; Parker, A.M.; Matar, R.M.; Gottbrecht, M.F.; Johansen, M.C.; Adams, M.P.; Griffiths, L.A.; Dunn, S.P.; Bidwell, K.L.; Menon, V.; et al. C-GRApH: A Validated Scoring System for Early Stratification of Neurologic Outcome after Out-of-Hospital Cardiac Arrest Treated with Targeted Temperature Management. J. Am. Heart Assoc. 2017, 6, e003821. [Google Scholar] [CrossRef]
- Grunau, B.; Reynolds, J.C.; Scheuermeyer, F.X.; Stenstrom, R.; Pennington, S.; Cheung, C.; Li, J.; Habibi, M.; Ramanathan, K.; Barbic, D.; et al. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: Informing minimum durations of resuscitation. Resuscitation 2016, 101, 50–56. [Google Scholar] [CrossRef]
- McKenzie, N.; Ball, S.; Bailey, P.; Finn, L.; Arendts, G.; Celenza, A.; Fatovich, D.; Jenkins, I.; Mukherjee, A.; Smedley, B.; et al. Neurological outcome in adult out-of-hospital cardiac arrest—Not all doom and gloom! Resuscitation 2021, 167, 227–232. [Google Scholar] [CrossRef]
- Nichol, G.; Thomas, E.; Callaway, C.W.; Hedges, J.; Powell, J.L.; Aufderheide, T.P.; Rea, T.; Lowe, R.; Brown, T.; Dreyer, J.; et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008, 300, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Barsan, W.G.; Levy, R.C. Experimental design for study of cardiopulmonary resuscitation in dogs. Ann. Emerg. Med. 1981, 10, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, T.; Tonon, C.R.; Martins, D.; Fávero, E.L., Jr.; Baumgratz, T.D.; Pereira, F.W.L.; Pinheiro, V.R.; Ballarin, R.S.; Queiroz, D.A.R.; Azevedo, P.S.; et al. Post-Cardiac Arrest: Mechanisms, Management, and Future Perspectives. J. Clin. Med. 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Stub, D.; Bernard, S.; Duffy, S.J.; Kaye, D.M. Post cardiac arrest syndrome: A review of therapeutic strategies. Circulation 2011, 123, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Gaisendrees, C.; Pooth, J.S.; Luehr, M.; Sabashnikov, A.; Yannopoulos, D.; Wahlers, T. Extracorporeal Cardiopulmonary Resuscitation—Evidence and Implications. Dtsch. Arztebl. Int. 2023, Forthcoming. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.J.; Granfeldt, A.; Guerguerian, A.M.; Sandroni, C.; Hsu, C.H.; Gardner, R.M.; Lind, P.C.; Eggertsen, M.A.; Johannsen, C.M.; Andersen, L.W. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: An updated systematic review. Resuscitation 2023, 182, 109665. [Google Scholar] [CrossRef]
- Scquizzato, T.; Bonaccorso, A.; Swol, J.; Gamberini, L.; Scandroglio, A.M.; Landoni, G.; Zangrillo, A. Refractory out-of-hospital cardiac arrest and extracorporeal cardiopulmonary resuscitation: A meta-analysis of randomized trials. Artif. Organs 2023, 47, 806–816. [Google Scholar] [CrossRef]
- Haas, N.L.; Coute, R.A.; Hsu, C.H.; Cranford, J.A.; Neumar, R.W. Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest-An ELSO registry study. Resuscitation 2017, 119, 56–62. [Google Scholar] [CrossRef]
- Bartos, J.A.; Grunau, B.; Carlson, C.; Duval, S.; Ripeckyj, A.; Kalra, R.; Raveendran, G.; John, R.; Conterato, M.; Frascone, R.J.; et al. Improved Survival with Extracorporeal Cardiopulmonary Resuscitation Despite Progressive Metabolic Derangement Associated with Prolonged Resuscitation. Circulation 2020, 141, 877–886. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, T.; Ro, Y.S.; Ahn, K.O.; Lee, E.J.; Hwang, S.S.; Song, S.W.; Song, K.J.; Shin, S.D. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: A propensity score-matched analysis. Resuscitation 2016, 99, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lunz, D.; Calabrò, L.; Belliato, M.; Contri, E.; Broman, L.M.; Scandroglio, A.M.; Patricio, D.; Malfertheiner, M.; Creteur, J.; Philipp, A.; et al. Extracorporeal membrane oxygenation for refractory cardiac arrest: A retrospective multicenter study. Intensive Care Med. 2020, 46, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horák, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Suverein, M.M.; Delnoij, T.S.R.; Lorusso, R.; Brandon Bravo Bruinsma, G.J.; Otterspoor, L.; Elzo Kraemer, C.V.; Vlaar, A.P.J.; van der Heijden, J.J.; Scholten, E.; den Uil, C.; et al. Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2023, 388, 299–309. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engström, A.E.; Lagrand, W.K.; Cherpanath, T.G.V.; Driessen, A.H.G.; de Mol, B.; Henriques, J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef]
- Shin, T.G.; Jo, I.J.; Sim, M.S.; Song, Y.B.; Yang, J.H.; Hahn, J.Y.; Choi, S.H.; Gwon, H.C.; Jeon, E.S.; Sung, K.; et al. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int. J. Cardiol. 2013, 168, 3424–3430. [Google Scholar] [CrossRef]
- Lamhaut, L.; Hutin, A.; Puymirat, E.; Jouan, J.; Raphalen, J.H.; Jouffroy, R.; Jaffry, M.; Dagron, C.; An, K.; Dumas, F.; et al. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: An observational study and propensity analysis. Resuscitation 2017, 117, 109–117. [Google Scholar] [CrossRef]
- Wengenmayer, T.; Rombach, S.; Ramshorn, F.; Biever, P.; Bode, C.; Duerschmied, D.; Staudacher, D.L. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit. Care 2017, 21, 157. [Google Scholar] [CrossRef]
- Inoue, A.; Hifumi, T.; Sakamoto, T.; Kuroda, Y. Extracorporeal Cardiopulmonary Resuscitation for Out-of-Hospital Cardiac Arrest in Adult Patients. J. Am. Heart Assoc. 2020, 9, e015291. [Google Scholar] [CrossRef]
- Ko, R.E.; Huh, K.; Kim, D.H.; Na, S.J.; Chung, C.R.; Cho, Y.H.; Jeon, K.; Suh, G.Y.; Yang, J.H. Nosocomial infections in in-hospital cardiac arrest patients who undergo extracorporeal cardiopulmonary resuscitation. PLoS ONE 2020, 15, e0243838. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Sawano, H.; Natsukawa, T.; Matsuoka, R.; Nakashima, T.; Takahagi, M.; Hayashi, Y. D-dimer predicts bleeding complication in out-of-hospital cardiac arrest resuscitated with ECMO. Am. J. Emerg. Med. 2018, 36, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Vaquer, S.; de Haro, C.; Peruga, P.; Oliva, J.C.; Artigas, A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann. Intensive Care 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Cavayas, Y.A.; Del Sorbo, L.; Munshi, L.; Sampson, C.; Fan, E. Intracranial hemorrhage on extracorporeal membrane oxygenation: An international survey. Perfusion 2021, 36, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sandersjöö, A.; Bartek, J., Jr.; Thelin, E.P.; Eriksson, A.; Elmi-Terander, A.; Broman, M.; Bellander, B.M. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: An observational cohort study. J. Intensive Care 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Gaisendrees, C.; Ivanov, B.; Gerfer, S.; Sabashnikov, A.; Eghbalzadeh, K.; Schlachtenberger, G.; Avgeridou, S.; Rustenbach, C.; Merkle, J.; Adler, C.; et al. Predictors of acute kidney injury in patients after extracorporeal cardiopulmonary resuscitation. Perfusion 2023, 38, 292–298. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.A.; Martin, C.; Raveendran, G.; Missov, E.; Conterato, M.; Frascone, R.J.; Trembley, A.; Sipprell, K.; John, R.; et al. Minnesota Resuscitation Consortium’s Advanced Perfusion and Reperfusion Cardiac Life Support Strategy for Out-of-Hospital Refractory Ventricular Fibrillation. J. Am. Heart Assoc. 2016, 5, e003732. [Google Scholar] [CrossRef]
- Tjelmeland, I.B.M.; Wnent, J.; Masterson, S.; Kramer-Johansen, J.; Ong, M.E.H.; Smith, K.; Skogvoll, E.; Lefering, R.; Lim, S.L.; Liu, N.; et al. Did lockdown influence bystanders’ willingness to perform cardiopulmonary resuscitation? A worldwide registry-based perspective. Resuscitation 2023, 186, 109764. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Kim, H.; Cho, Y.H. Role of extracorporeal cardiopulmonary resuscitation in adults. Acute Crit. Care 2020, 35, 1–9. [Google Scholar] [CrossRef]
- Soar, J.; Maconochie, I.; Wyckoff, M.H.; Olasveengen, T.M.; Singletary, E.M.; Greif, R.; Aickin, R.; Bhanji, F.; Donnino, M.W.; Mancini, M.E.; et al. 2019 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation 2019, 140, e826–e880. [Google Scholar] [PubMed]
- Richardson, A.S.C.; Tonna, J.E.; Nanjayya, V.; Nixon, P.; Abrams, D.C.; Raman, L.; Bernard, S.; Finney, S.J.; Grunau, B.; Youngquist, S.T.; et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 221–228. [Google Scholar] [CrossRef]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Ubben, J.F.H.; Heuts, S.; Delnoij, T.S.R.; Suverein, M.M.; van de Koolwijk, A.F.; van der Horst, I.C.C.; Maessen, J.G.; Bartos, J.; Kavalkova, P.; Rob, D.; et al. Extracorporeal cardiopulmonary resuscitation for refractory OHCA: Lessons from three randomized controlled trials-the trialists’ view. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Greif, D.M.; Eichmann, A. Vascular biology: Brain vessels squeezed to death. Nature 2014, 508, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Trummer, G.; Beyersdorf, F.; Scherer, C.; Förster, K.; Taunyane, I.; Benk, C. Improved Outcome in an Animal Model of Prolonged Cardiac Arrest Through Pulsatile High Pressure Controlled Automated Reperfusion of the Whole Body. Artif. Organs 2018, 42, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Shariff, M.; Dobariya, A.; Albaghdadi, O.; Awkal, J.; Moussa, H.; Reyes, G.; Syed, M.; Hart, R.; Longfellow, C.; Douglass, D.; et al. Maintenance of pig brain function under extracorporeal pulsatile circulatory control (EPCC). Sci. Rep. 2023, 13, 13942. [Google Scholar] [CrossRef]
- Okamoto, F.; Allen, B.S.; Buckberg, G.D.; Bugyi, H.; Leaf, J. Reperfusion conditions: Importance of ensuring gentle versus sudden reperfusion during relief of coronary occlusion. J. Thorac. Cardiovasc. Surg. 1986, 92 Pt 2, 613–620. [Google Scholar] [CrossRef]
- Hossmann, K.A. Resuscitation potentials after prolonged global cerebral ischemia in cats. Crit. Care Med. 1988, 16, 964–971. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Møller, J.E.; Schmidt, H.; Grand, J.; Mølstrøm, S.; Borregaard, B.; Venø, S.; Sarkisian, L.; Mamaev, D.; Jensen, L.O.; et al. Blood-Pressure Targets in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 387, 1456–1466. [Google Scholar] [CrossRef]
- Senthil, K.; Morgan, R.W.; Hefti, M.M.; Karlsson, M.; Lautz, A.J.; Mavroudis, C.D.; Ko, T.; Nadkarni, V.M.; Ehinger, J.; Berg, R.A.; et al. Haemodynamic-directed cardiopulmonary resuscitation promotes mitochondrial fusion and preservation of mitochondrial mass after successful resuscitation in a pediatric porcine model. Resusc. Plus 2021, 6, 100124. [Google Scholar] [CrossRef] [PubMed]
- Hossmann, K.A.; Schmidt-Kastner, R.; Grosse Ophoff, B. Recovery of integrative central nervous function after one hour global cerebro-circulatory arrest in normothermic cat. J. Neurol. Sci. 1987, 77, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Waaben, J.; Husum, B.; Voldby, B.; Bødker, A.; Hansen, A.J.; Gjedde, A. Nonpulsatile cardiopulmonary bypass disrupts the flow-metabolism couple in the brain. J. Thorac. Cardiovasc. Surg. 1985, 90, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, P.; Sipkema, P.; Westerhof, N. Pump perfusion abolishes autoregulation possibly via prostaglandin release. Am. J. Physiol. 1988, 255 Pt 2, H280–H287. [Google Scholar] [CrossRef]
- Mendelowitz, D.; Scher, A.M. Pulsatile pressure can prevent rapid baroreflex resetting. Am. J. Physiol. 1990, 258 Pt 2, H92–H100. [Google Scholar] [CrossRef]
- Iijima, T.; Bauer, R.; Hossmann, K.A. Brain resuscitation by extracorporeal circulation after prolonged cardiac arrest in cats. Intensive Care Med. 1993, 19, 82–88. [Google Scholar] [CrossRef]
- Kumar, K.; Goodrich, J.; Marcoux, F. Comparison of vascular perfusion in ischemia-sensitive and ischemia-resistant regions of gerbil brain by an automated laser cytometric device: A preliminary study. J. Neurosci. Methods 1991, 39, 1–8. [Google Scholar] [CrossRef]
- Shiroyama, Y.; Nagamitsu, T.; Yamashita, K.; Yamashita, T.; Abiko, S.; Ito, H. Changes in brain stem blood flow under various grades of brain stem ischemia. Tohoku J. Exp. Med. 1991, 164, 237–246. [Google Scholar] [CrossRef]
- Pappalardo, F.; Montisci, A. What is extracorporeal cardiopulmonary resuscitation? J. Thorac. Dis. 2017, 9, 1415–1419. [Google Scholar] [CrossRef]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Deakin, C.D.; Soar, J.; Böttiger, B.W.; Smith, G. European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation 2005, 67 (Suppl. S1), S39–S86. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef]
- Arrich, J.; Schütz, N.; Oppenauer, J.; Vendt, J.; Holzer, M.; Havel, C.; Herkner, H. Hypothermia for neuroprotection in adults after cardiac arrest. Cochrane Database Syst. Rev. 2023, 5, Cd004128. [Google Scholar]
- Fernando, S.M.; Di Santo, P.; Sadeghirad, B.; Lascarrou, J.B.; Rochwerg, B.; Mathew, R.; Sekhon, M.S.; Munshi, L.; Fan, E.; Brodie, D.; et al. Targeted temperature management following out-of-hospital cardiac arrest: A systematic review and network meta-analysis of temperature targets. Intensive Care Med. 2021, 47, 1078–1088. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Lanzafame, B.; Dezio, V.; Busalacchi, D.; Messina, A.; Ristagno, G.; Pelosi, P.; Astuto, M. Targeted Temperature Management after Cardiac Arrest: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. J. Clin. Med. 2021, 10, 3943. [Google Scholar] [CrossRef]
- Sterz, F.; Safar, P.; Tisherman, S.; Radovsky, A.; Kuboyama, K.; Oku, K. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Crit. Care Med. 1991, 19, 379–389. [Google Scholar] [CrossRef]
- Kuboyama, K.; Safar, P.; Radovsky, A.; Tisherman, S.A.; Stezoski, S.W.; Alexander, H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: A prospective, randomized study. Crit. Care Med. 1993, 21, 1348–1358. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cochemé, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Shou, B.L.; Ong, C.S.; Premraj, L.; Brown, P.; Tonna, J.E.; Dalton, H.J.; Kim, B.S.; Keller, S.P.; Whitman, G.J.R.; Cho, S.M. Arterial oxygen and carbon dioxide tension and acute brain injury in extracorporeal cardiopulmonary resuscitation patients: Analysis of the extracorporeal life support organization registry. J. Heart Lung Transplant. 2023, 42, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, G.F.; Barth, A.S. Sudden cardio arrest: Oxidative stress irritates the heart. Nat. Med. 2010, 16, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Cheskes, S.; Verbeek, P.R.; Drennan, I.R.; McLeod, S.L.; Turner, L.; Pinto, R.; Feldman, M.; Davis, M.; Vaillancourt, C.; Morrison, L.J.; et al. Defibrillation Strategies for Refractory Ventricular Fibrillation. N. Engl. J. Med. 2022, 387, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Alternative defibrillation strategies improve outcomes. Nat. Rev. Cardiol. 2023, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, F.; Acar, C.; Buckberg, G.D.; Partington, M.T.; Okamoto, F.; Allen, B.S.; Bugyi, H.I.; Young, H.H. Studies on prolonged acute regional ischemia. IV. Aggressive surgical treatment for intractable ventricular fibrillation after acute myocardial infarction. J. Thorac. Cardiovasc. Surg. 1989, 98, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, O.J.; Allen, B.S.; Buckberg, G.D.; Hristov, N.; Tan, Z.; Villablanca, J.P.; Trummer, G. Resuscitation after prolonged cardiac arrest: Role of cardiopulmonary bypass and systemic hyperkalemia. Ann. Thorac. Surg. 2010, 89, 1972–1979. [Google Scholar] [CrossRef]
- Athanasuleas, C.L.; Buckberg, G.D.; Allen, B.S.; Beyersdorf, F.; Kirsh, M.M. Sudden cardiac death: Directing the scope of resuscitation towards the heart and brain. Resuscitation 2006, 70, 44–51. [Google Scholar] [CrossRef]
- Beyersdorf, F.; Kirsh, M.; Buckberg, G.D.; Allen, B.S. Warm glutamate/aspartate-enriched blood cardioplegic solution for perioperative sudden death. J. Thorac. Cardiovasc. Surg. 1992, 104, 1141–1147. [Google Scholar] [CrossRef]
- Wengenmayer, T.; Schroth, F.; Biever, P.M.; Duerschmied, D.; Benk, C.; Trummer, G.; Kaier, K.; Bode, C.; Staudacher, D.L. Albumin fluid resuscitation in patients on venoarterial extracorporeal membrane oxygenation (VA-ECMO) therapy is associated with improved survival. Intensive Care Med. 2018, 44, 2312–2314. [Google Scholar] [CrossRef]
- Oldman, A.H.; Martin, D.S.; Feelisch, M.; Grocott, M.P.W.; Cumpstey, A.F. Effects of perioperative oxygen concentration on oxidative stress in adult surgical patients: A systematic review. Br. J. Anaesth. 2021, 126, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Cabello, J.B.; Burls, A.; Emparanza, J.I.; Bayliss, S.E.; Quinn, T. Oxygen therapy for acute myocardial infarction. Cochrane Database Syst. Rev. 2016, 12, Cd007160. [Google Scholar] [CrossRef] [PubMed]
- Kilgannon, J.H.; Jones, A.E.; Shapiro, N.I.; Angelos, M.G.; Milcarek, B.; Hunter, K.; Parrillo, J.E.; Trzeciak, S. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. Jama 2010, 303, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Koksal, G.M.; Dikmen, Y.; Erbabacan, E.; Aydin, S.; Çakatay, U.; Sitar, M.E.; Altindas, F. Hyperoxic oxidative stress during abdominal surgery: A randomized trial. J. Anesthesia 2016, 30, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front. Biosci. 2008, 13, 6653–6661. [Google Scholar] [CrossRef]
- Kilgannon, J.H.; Jones, A.E.; Parrillo, J.E.; Dellinger, R.P.; Milcarek, B.; Hunter, K.; Shapiro, N.I.; Trzeciak, S. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation 2011, 123, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, J.; Weatherall, M.; Shirtcliffe, P.; Bellomo, R.; Young, P.; Beasley, R. The effect of hyperoxia following cardiac arrest—A systematic review and meta-analysis of animal trials. Resuscitation 2012, 83, 417–422. [Google Scholar] [CrossRef]
- Roberts, B.W.; Kilgannon, J.H.; Hunter, B.R.; Puskarich, M.A.; Pierce, L.; Donnino, M.; Leary, M.; Kline, J.A.; Jones, A.E.; Shapiro, N.I.; et al. Association Between Early Hyperoxia Exposure After Resuscitation From Cardiac Arrest and Neurological Disability: Prospective Multicenter Protocol-Directed Cohort Study. Circulation 2018, 137, 2114–2124. [Google Scholar] [CrossRef]
- Schmidt, H.; Kjaergaard, J.; Hassager, C.; Mølstrøm, S.; Grand, J.; Borregaard, B.; Roelsgaard Obling, L.E.; Venø, S.; Sarkisian, L.; Mamaev, D.; et al. Oxygen Targets in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 387, 1467–1476. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Johansson, P.I.; Kjaergaard, J.; Wanscher, M.; Ostrowski, S.R.; Bjerre, M.; Hassager, C. Level of systemic inflammation and endothelial injury is associated with cardiovascular dysfunction and vasopressor support in post-cardiac arrest patients. Resuscitation 2017, 121, 179–186. [Google Scholar] [CrossRef]
- Wiberg, S.; Stride, N.; Bro-Jeppesen, J.; Holmberg, M.J.; Kjærgaard, J.; Larsen, S.; Donnino, M.W.; Hassager, C.; Dela, F. Mitochondrial dysfunction in adults after out-of-hospital cardiac arrest. Eur. Heart J. Acute Cardiovasc. Care 2020, 9 (Suppl. S4), S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Gao, X.; Dilger, J.P.; Lin, J. Neuroprotective effect of lidocaine: Is there clinical potential? Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 9–13. [Google Scholar] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004, 61, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.S.; Yang, J.H.; Cho, Y.H.; Chung, C.R.; Park, C.M.; Jeon, K.; Suh, G.Y. Clinical outcomes after rescue extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest. Emerg. Med. J. 2017, 34, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ahn, S.; Sohn, C.H.; Seo, D.W.; Lee, Y.S.; Lee, J.H.; Oh, B.J.; Lim, K.S.; Kim, W.Y. Long-term neurological outcomes in patients after out-of-hospital cardiac arrest. Resuscitation 2016, 101, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Friess, S.H.; Sutton, R.M.; French, B.; Bhalala, U.; Maltese, M.R.; Naim, M.Y.; Bratinov, G.; Arciniegas Rodriguez, S.; Weiland, T.R.; Garuccio, M.; et al. Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation 2014, 85, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.M.; Berg, R.A.; Otto, C.W. Monitoring during cardiac arrest: Are we there yet? Curr. Opin. Crit. Care 2003, 9, 211–217. [Google Scholar] [CrossRef]
- Kleinman, M.E.; Perkins, G.D.; Bhanji, F.; Billi, J.E.; Bray, J.E.; Callaway, C.W.; de Caen, A.; Finn, J.C.; Hazinski, M.F.; Lim, S.H.; et al. ILCOR Scientific Knowledge Gaps and Clinical Research Priorities for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: A Consensus Statement. Resuscitation 2018, 127, 132–146. [Google Scholar] [CrossRef]
- Maciel, C.B.; Barden, M.M.; Greer, D.M. Neurologic Recovery After Cardiac Arrest: A Multifaceted Puzzle Requiring Comprehensive Coordinated Care. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 52. [Google Scholar] [CrossRef]
- Sutton, R.M.; Friess, S.H.; Naim, M.Y.; Lampe, J.W.; Bratinov, G.; Weiland, T.R., 3rd; Garuccio, M.; Nadkarni, V.M.; Becker, L.B.; Berg, R.A. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am. J. Respir. Crit. Care Med. 2014, 190, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.B.; Mendes, P.V.; Hirota, A.S.; Barbosa, E.V.; Maciel, A.T.; Schettino, G.P.; Costa, E.L.; Azevedo, L.C.; Park, M. Severe hypoxemia during veno-venous extracorporeal membrane oxygenation: Exploring the limits of extracorporeal respiratory support. Clinics 2014, 69, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Beyea, M.M.; Tillmann, B.W.; Iansavichene, A.E.; Randhawa, V.K.; Van Aarsen, K.; Nagpal, A.D. Neurologic outcomes after extracorporeal membrane oxygenation assisted CPR for resuscitation of out-of-hospital cardiac arrest patients: A systematic review. Resuscitation 2018, 130, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Han, K.S.; Kim, S.J.; Lee, E.J.; Jung, J.S.; Park, J.H.; Lee, S.W. Experience of extracorporeal cardiopulmonary resuscitation in a refractory cardiac arrest patient at the emergency department. Clin. Cardiol. 2019, 42, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Deballon, I.; Hornby, L.; Shemie, S.D.; Bhanji, F.; Guadagno, E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation 2016, 101, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.C.; Anderson, M.L.; Bruder, E.; Daya, M.R.; Gaffney, A.; Otto, C.W.; Singer, A.J.; Thiagarajan, R.R.; Travers, A.H. Part 6: Alternative Techniques and Ancillary Devices for Cardiopulmonary Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132 (Suppl. S2), S436–S443. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Rodriguez-Sinovas, A.; Ruiz-Meana, M.; Inserte, J.; Agulló, L.; Cabestrero, A. The end-effectors of preconditioning protection against myocardial cell death secondary to ischemia-reperfusion. Cardiovasc. Res. 2006, 70, 274–285. [Google Scholar] [CrossRef]
- Beyersdorf, F.; Unger, A.; Wildhirt, A.; Kretzer, U.; Deutschländer, N.; Krüger, S.; Matheis, G.; Hanselmann, A.; Zimmer, G.; Satter, P. Studies of reperfusion injury in skeletal muscle: Preserved cellular viability after extended periods of warm ischemia. J. Cardiovasc. Surg. 1991, 32, 664–676. [Google Scholar]
- Buckberg, G.D. Studies Of Controlled Reperfusion After Ischemia: I. When is cardiac muscle damaged irreversibly? J. Thorac. Cardiovasc. Surg. 1986, 92, 483–487. [Google Scholar] [CrossRef]
- Haab, F.; Julia, P.; Nochy, D.; Cambillau, M.; Fabiani, J.N.; Thibault, P. Improvement of postischemic renal function by limitation of initial reperfusion pressure. J. Urol. 1996, 155, 1089–1093. [Google Scholar] [CrossRef]
- Halldorsson, A.O.; Kronon, M.; Allen, B.S.; Rahman, S.; Wang, T.; Layland, M.; Sidle, D. Controlled reperfusion prevents pulmonary injury after 24 hours of lung preservation. Ann. Thorac. Surg. 1998, 66, 877–884; discussion 884–885. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.S.; Ko, Y.; Buckberg, G.D.; Tan, Z. Studies of isolated global brain ischaemia: III. Influence of pulsatile flow during cerebral perfusion and its link to consistent full neurological recovery with controlled reperfusion following 30 min of global brain ischaemia. Eur. J. Cardio-Thoracic Surg. 2012, 41, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.S.; Ko, Y.; Buckberg, G.D.; Tan, Z. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia—The importance of perfusion pressure. Eur. J. Cardio-Thoracic Surg. 2012, 41, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Foerster, K.; Benk, C.; Beyersdorf, F.; Cristina Schmitz, H.; Wittmann, K.; Taunyane, I.; Heilmann, C.; Trummer, G. Twenty minutes of normothermic cardiac arrest in a pig model: The role of short-term hypothermia for neurological outcome. Perfusion 2018, 33, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Foerster, K.; D’Inka, M.; Beyersdorf, F.; Benk, C.; Nguyen-Thanh, T.; Mader, I.; Fritsch, B.; Ihling, C.; Mueller, K.; Heilmann, C.; et al. Prolonged cardiac arrest and resuscitation by extracorporeal life support: Favourable outcome without preceding anticoagulation in an experimental setting. Perfusion 2013, 28, 520–528. [Google Scholar] [CrossRef]
- Trummer, G.; Foerster, K.; Buckberg, G.D.; Benk, C.; Heilmann, C.; Mader, I.; Feuerhake, F.; Liakopoulos, O.; Brehm, K.; Beyersdorf, F. Successful resuscitation after prolonged periods of cardiac arrest: A new field in cardiac surgery. J. Thorac. Cardiovasc. Surg. 2010, 139, 1325–1332.e2. [Google Scholar] [CrossRef]
- Trummer, G.; Foerster, K.; Buckberg, G.D.; Benk, C.; Mader, I.; Heilmann, C.; Liakopoulos, O.; Beyersdorf, F. Superior neurologic recovery after 15 minutes of normothermic cardiac arrest using an extracorporeal life support system for optimized blood pressure and flow. Perfusion 2014, 29, 130–138. [Google Scholar] [CrossRef]
- Allen, B.S.; Castellá, M.; Buckberg, G.D.; Tan, Z. Conditioned blood reperfusion markedly enhances neurologic recovery after prolonged cerebral ischemia. J. Thorac. Cardiovasc. Surg. 2003, 126, 1851–1858. [Google Scholar] [CrossRef]


| Characteristic | Parameter | Value |
|---|---|---|
| Age | [years], mean ± SD Range | 59.0 ± 13.4 21–86 |
| 18–64 years, n (%) | 46 (67) | |
| 65–75 years, n (%) | 18 (26) | |
| Sex | Male, n (%) | 54 (78.3) |
| Female, n (%) | 15 (21.7) | |
| Weight | [kg], median (IQR) (n = 56) | 84 (80–96) |
| Medical history | Previous illnesses known, n (%) | 55 (79.7) |
| Coronary artery disease, n (%) | 26 (37.7) | |
| Cardiomyopathy, n (%) | 8 (11.6) | |
| Cerebrovascular stenoses, n (%) | 3 (4.3) | |
| Other cardiovascular diseases, n (%) | 18 (26.1) | |
| Diabetes, n (%) | 10 (14.5) | |
| Respiratory, n (%) | 3 (4.3) | |
| Substance abuse, n (%) | 14 (20.3) | |
| Renal insufficiency, n (%) | 5 (7.2) | |
| Cancer, n (%) | 4 (5.8) | |
| Rheumatic, n (%) | 2 (2.9) | |
| Other, n (%) | 31 (44.9) | |
| Etiology of CA | Myocardial infarction, n (%) | 43 (62.3) |
| Aortic dissection type A, n (%) | 7 (10.1) | |
| Pulmonary arterial embolism, n (%) | 4 (5.8) | |
| Arrhythmogenic, n (%) | 4 (5.8) | |
| Cardiomyopathy, n (%) | 3 (4.3) | |
| Valvular heart disease (aortic stenosis), n (%) | 1 (1.4) | |
| Hypoxia, n (%) | 3 (4.3) | |
| Intoxication, n (%) | 2 (2.9) | |
| Drowning, n (%) | 1 (1.4) | |
| Hypovolemia, n (%) | 1 (1.4) | |
| Implanted AED | Internal defibrillator, n (%) | 2 (2.9) |
| External defibrillator (vest), n (%) | 0 (0) | |
| Location of CA | IHCA, n (%) | 29 (42) |
| OHCA, n (%) | 40 (58) | |
| Medical facility, n (%) | 2 (5.0) | |
| Home/residence, n (%) | 13 (32.5) | |
| Street/highway, n (%) | 9 (22.5) | |
| Industrial/workplace, n (%) | 6 (15.0) | |
| Sports/recreation event, n (%) | 5 (12.5) | |
| Public building, n (%) | 3 (7.5) | |
| Other, n (%) | 2 (5.0) | |
| Unwitnessed CA | n (%) | 8 (11.6) |
| Witnessed CA | Bystander witnessed, n (%) | 29 (42.0) |
| Of those, bystander CPR, n (%) | 22 (75.9) | |
| EMS witnessed, n (%) | 32 (46.4) | |
| AED use among n = 22 patients | AED used | 1 (4.5) |
| Initial rhythm monitored at CA in n = 62 patients | Ventricular fibrillation, n (%) | 29 (46.8) |
| Pulseless ventricular tachycardia, n (%) | 4 (6.5) | |
| Asystole, n (%) | 10 (16.1) | |
| Pulseless electrical activity, n (%) | 19 (30.6) | |
| Airway management in n = 64 patients | Endotracheal tube, n (%) | 58 (90.6) |
| Supraglottic airway, n (%) | 3 (4.7) | |
| Surgical airway, n (%) | 2 (3.1) | |
| Not used, n (%) | 1 (1.6) | |
| Mechanical CPR device used in n = 56 patients | n (%) | 36 (64.3) |
| Defibrillations in n = 49 patients | [Number per patient], median (IQR) | 3 (IQR 0–5) |
| End-tidal CO2 during CPR | [mmHg], median (IQR) (n = 11) | 25 (IQR 20–30) |
| pH before CARL * | Mean ± SD (n = 21) | 7.0 ± 0.3 |
| Lactate before CARL * | [mmol/L], median (IQR) (n = 21) | 10.1 (7.7–12.3) |
| Characteristic | Parameter | Value |
|---|---|---|
| IHCA + OHCA | Overall survival at hospital discharge, n (%) | 29 (42) |
| Deemed “non-survivable”, n (%) | 9 (13) | |
| Survival among remaining, n (%) | 29 (48) | |
| Survival at 3 months with CPC 1–2, n (%) | 23 (33) | |
| IHCA (n = 29) | Overall survival at hospital discharge, n (%) | 15 (52) |
| Survival at 3 months with CPC 1–2, n (%) | 12 (41) | |
| OHCA (n = 40) | Overall survival at hospital discharge, n (%) | 14 (35) |
| Survival at 3 months with CPC 1–2, n (%) | 11 (28) | |
| Survival at hospital discharge of patients | ||
| transported to the hospital (n = 26), n (%) | 6 (23) | |
| Survival at hospital discharge of patients | ||
| cannulated pre-hospital (n = 14), n (%) | 8 (57) | |
| Primary cause of death among n = 40 deceased | Neurological, n (%) | 10 (25.0) |
| Multiple organ failure, n (%) | 11 (27.5) | |
| Hemorrhage, n (%) | 3 (7.5) | |
| Cardiac pump failure, n (%) | 4 (10.0) | |
| Acute aortic dissection type A, n (%) | 7 (17.5) | |
| Left ventricular rupture, n (%) | 2 (5.0) | |
| Sepsis, n (%) | 0 (0) | |
| Other, n (%) | 3 (7.5) | |
| ICU stay (n = 62) | [days], median (IQR) | 8.1 (1.5–23.0) |
| Hospital stay | [days], median (IQR) | 10 (1–28) |
| Among deceased patients [days], median (IQR) (n = 40) | 1 (0–6) | |
| Among survivors [days], median (IQR) (n = 29) | 28 (16–49) |
| Time Interval | Number of Patients * | Value |
|---|---|---|
| Time from emergency call to EMS arrival (OHCA) [min], mean ± SD | 26/40 | 8.9 ± 4.9 |
| Time from call to first shock [min], median (IQR) | 15/69 | 12.4 (6.5–19.5) |
| Duration of CCPR in all patients [min], median (IQR) | ||
| IHCA + OHCA | 62/69 | 51.5 (30.0–74.5) |
| IHCA | 26/62 | 33.5 (18.5–49.3) |
| OHCA | 36/62 | 68.5 (43.8–81.3) |
| Pre-hospital cannulation | 11/36 | 31.0 (20.0–56.5) |
| In-hospital cannulation | 25/36 | 75.0 (67.0–88.0) |
| Duration of CCPR only for OHCA, n (%) | ||
| <30 min | 36 | 5 (13.9) |
| 30–60 min | 36 | 9 (25.0) |
| >60 min | 36 | 22 (61.1) |
| Time until cannulation was established (IHCA and OHCA), n (%) | ||
| <10 min | 41 | 4 (9.8) |
| 10–15 min | 41 | 19 (46.3) |
| 16–20 min | 41 | 6 (14.6) |
| 21–25 min | 41 | 6 (14.6) |
| >25 min | 41 | 6 (14.6) |
| Time from CA to start of CARL (IHCA + OHCA) [min], mean ± SD | 54/69 | 59.2 ± 30.8 |
| Organ System | 24 h | 7 Days | 30 Days | Hospital Discharge |
|---|---|---|---|---|
| Number of patients assessed at timepoint | 44 | 32 | 18 | 23 |
| Kidney | ||||
| Creatinine [mg/dL], median (IQR) | 1.44 (1.03–1.99) | 1.30 (0.96–2.65) | 0.98 (0.75–1.82) | 0.75 (0.65–0.95) |
| Patients with data, n (total) | 32 | 29 | 18 | 19 |
| GFR [mL/min], mean ± SD | 58.2 ± 22.5 | 63.7 ± 31.5 | 78.9 ± 52.0 | 82.7 ± 32.6 |
| Patients with data, n (total) | 20 | 15 | 7 | 9 |
| Renal replacement therapy, n (%) | 4 (9.8) | 9 (28.1) | 2 (11.1) | 1 (4.3) |
| Patients with data, n (total) | 41 | 32 | 18 | 23 |
| Liver | ||||
| AST/GOT [U/L], median (IQR) | 273 (165–384) | 101 (70–312) | 43 (34–70) | 25 (21–37) |
| Patients with data, n (total) | 31 | 20 | 9 | 9 |
| ALT/GPT [U/L], median (IQR) | 103 (49–195) | 69 (60–144) | 5 (53–102) | 31 (22–86) |
| Patients with data, n (total) | 32 | 18 | 11 | 11 |
| Respiratory System | ||||
| Invasive ventilation, n (%) * | 44 (100) | 20 (63) | 6 (33) | 3 (13) |
| Patients with data, n (total) | 44 | 32 | 18 | 23 |
| Non-invasive ventilation, n (%) | 0 (0) | 2 (6) | 1 (6) | 0 (0) |
| Patients with data, n (total) | 44 | 32 | 18 | 23 |
| Cardiovascular System | ||||
| LV functional impairment | ||||
| None, n (%) | 4 (9.1) | 11 (34.4) | 4 (22.2) | 8 (34.8) |
| Mild, n (%) ** | 1 (2.3) | 1 (3.1) | 0 (0) | 3 (13.0) |
| Moderate, n (%) ** | 7 (15.9) | 1 (3.1) | 0 (0) | 1 (4.3) |
| Severe, n (%) ** | 19 (43.2) | 7 (21.9) | 4 (22.2) | 3 (13.0) |
| No values available, n (%) | 13 (29.5) | 12 (37.5) | 10 (5.6) | 8 (34.8) |
| Patients with data, n (total) | 44 | 32 | 18 | 23 |
| Dependent on inotropes | 34 (79.1) | 9 (28.1) | 2 (11.1) | 1 (4.5) |
| Patients with data, n (total) | 43 | 32 | 18 | |
| Dependent on vasopressors | 39 (88.6) | 12 (37.5) | 2 (11.1) | 0 (0) |
| Patients with data, n (total) | 44 | 32 | 18 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trummer, G.; Benk, C.; Pooth, J.-S.; Wengenmayer, T.; Supady, A.; Staudacher, D.L.; Damjanovic, D.; Lunz, D.; Wiest, C.; Aubin, H.; et al. Treatment of Refractory Cardiac Arrest by Controlled Reperfusion of the Whole Body: A Multicenter, Prospective Observational Study. J. Clin. Med. 2024, 13, 56. https://doi.org/10.3390/jcm13010056
Trummer G, Benk C, Pooth J-S, Wengenmayer T, Supady A, Staudacher DL, Damjanovic D, Lunz D, Wiest C, Aubin H, et al. Treatment of Refractory Cardiac Arrest by Controlled Reperfusion of the Whole Body: A Multicenter, Prospective Observational Study. Journal of Clinical Medicine. 2024; 13(1):56. https://doi.org/10.3390/jcm13010056
Chicago/Turabian StyleTrummer, Georg, Christoph Benk, Jan-Steffen Pooth, Tobias Wengenmayer, Alexander Supady, Dawid L. Staudacher, Domagoj Damjanovic, Dirk Lunz, Clemens Wiest, Hug Aubin, and et al. 2024. "Treatment of Refractory Cardiac Arrest by Controlled Reperfusion of the Whole Body: A Multicenter, Prospective Observational Study" Journal of Clinical Medicine 13, no. 1: 56. https://doi.org/10.3390/jcm13010056