The Interpretation of Standard Cardiopulmonary Exercise Test Indices of Cardiac Function in Chronic Kidney Disease
Abstract
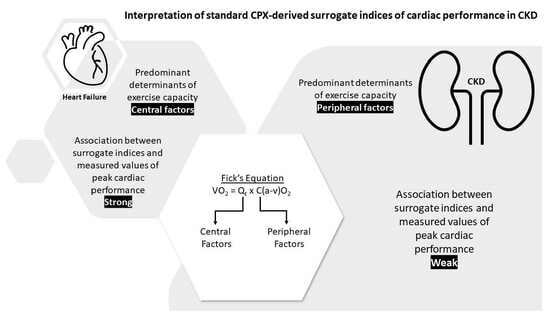
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Study Investigations
- Resting measures: The O2 consumption, CO2 production, respiratory rate, and cardiac output at rest were measured using a Medgraphics CardiO2 Analytic System (Medgraphics Corp., St. Paul, MN, USA). Resting cardiac output was calculated using the Collier CO2 rebreathing method [8,9]. The Collier’s equilibration method has been shown to have good correlation with thermodilution techniques at rest [10] and is easy to use, and therefore it was utilized for resting measurements.
- Determination of exercise capacity (VO2peak): Subjects then underwent an incremental exercise test on a treadmill according to a standard Bruce protocol (or modified Bruce protocol for HF patients). The speed and incline of the treadmill were increased every three minutes according to the protocol until the subjects reached volitional exhaustion. Throughout the treadmill test, O2 consumption, CO2 production, end-tidal partial pressure of CO2, tidal ventilation, and respiratory rate were measured using breath-to-breath analysis. Ventilatory (“anaerobic”) threshold was measured by the V-slope method [11]. A 12-lead ECG was monitored throughout, and the subject’s heart rate (HR) was obtained from this. Blood pressure was measured at every stage of the CPX test.
- Determination of peak cardiac output: A second treadmill test was performed after a rest period of at least 40 minutes. The first treadmill test also served as a familiarization step. The speed and incline of the treadmill were adjusted manually. The subjects exercised on the treadmill to 95% of their VO2peak as established in the incremental exercise test. Two or three cardiac output measurements were made using the Defare’s CO2 rebreathing method [12]. The Defare’s method was chosen because this method has been shown to correlate well with cardiac output obtained with thermodilution techniques during exercise [13]. The formulae used in the study are listed in Table 1. The blood pressure was measured using a sphygmomanometer after each determination of cardiac output.
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Association between the Peak SV and Peak O2-Pulse
3.3. Association between the Estimated and Measured Peak SV
3.4. Association between the AT and Peak Cardiac Power (CPOpeak)
3.5. Association between the Peak Circulatory Power, Peak Ventilatory Power, and Peak Cardiac Power in CKD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pella, E.; Boutou, A.; Boulmpou, A.; Papadopoulos, C.E.; Papagianni, A.; Sarafidis, P. Cardiopulmonary exercise testing in patients with end-stage kidney disease: Principles, methodology and clinical applications of the optimal tool for exercise tolerance evaluation. Nephrol. Dial Transpl. 2022, 37, 2335–2350. [Google Scholar] [CrossRef]
- Clyne, N.; Anding-Rost, K. Exercise training in chronic kidney disease-effects, expectations and adherence. Clin. Kidney J. 2021, 14, ii3–ii14. [Google Scholar] [CrossRef] [PubMed]
- Chinnappa, S.L.N.; Baldo, O.; Shih, M.; Tu, Y.K.; Mooney, A. Cardiac and Noncardiac Determinants of Exercise Capacity in CKD. J. Am. Soc. Nephrol. 2021, 32, 9. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Cattadori, G.; Apostolo, A.; Contini, M.; Palermo, P.; Marenzi, G.; Wasserman, K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: A new tool for heart failure evaluation. J. Am. Coll. Cardiol. 2005, 46, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Vignati, C.; Gentile, P.; Boiti, C.; Farina, S.; Salvioni, E.; Mapelli, M.; Magri, D.; Paolillo, S.; Corrieri, N.; et al. Reference Values for Peak Exercise Cardiac Output in Healthy Individuals. Chest 2017, 151, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.G.; Cooke, G.A.; Wright, D.J.; Parsons, W.J.; Riley, R.L.; Marshall, P.; Tan, L.B. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur. Heart J. 2001, 22, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.R. Determination of mixed venous CO2 tensions by rebreathing. J. Appl. Physiol. 1956, 9, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, L.; Defoor, J.; Schepers, D.; Brusselle, S.; Reybrouck, T.; Fagard, R. Comparison of cardiac output measured by two automated methods of CO2 rebreathing. Med. Sci. Sports Exerc. 2000, 32, 1028–1034. [Google Scholar] [CrossRef]
- Muiesan, G.; Sorbini, C.A.; Solinas, E.; Grassi, V.; Casucci, G.; Petz, E. Comparison of CO2-rebreathing and direct Fick methods for determining cardiac output. J. Appl. Physiol. 1968, 24, 424–429. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Defares, J.G. Determination of PvCO2 from the exponential CO2 rise during rebreathing. J. Appl. Physiol. 1958, 13, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Reybrouck, T.; Fagard, R. Assessment of cardiac output at rest and during exercise by a carbon dioxide rebreathing method. Eur. Heart J. 1990, 11 (Suppl. I), 21–25. [Google Scholar] [CrossRef] [PubMed]
- Accalai, E.; Vignati, C.; Salvioni, E.; Pezzuto, B.; Contini, M.; Cadeddu, C.; Agostoni, P. Non-invasive estimation of stroke volume during exercise from oxygen in heart failure patients. Eur. J. Prev. Cardiol. 2021, 28, 280–286. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing: What Is its Value? J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- Stringer, W.W.; Hansen, J.E.; Wasserman, K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J. Appl. Physiol. 1997, 82, 908–912. [Google Scholar] [CrossRef]
- Agostoni, P.G.; Wasserman, K.; Perego, G.B.; Guazzi, M.; Cattadori, G.; Palermo, P.; Lauri, G.; Marenzi, G. Non-invasive measurement of stroke volume during exercise in heart failure patients. Clin. Sci. 2000, 98, 545–551. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Chinnappa, S.; Hothi, S.S.; Tan, L.B. Is uremic cardiomyopathy a direct consequence of chronic kidney disease? Expert Rev. Cardiovasc. Ther. 2014, 12, 127–130. [Google Scholar] [CrossRef]
- Chinnappa, S.; White, E.; Lewis, N.; Baldo, O.; Tu, Y.K.; Glorieux, G.; Vanholder, R.; El Nahas, M.; Mooney, A. Early and asymptomatic cardiac dysfunction in chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 450–458. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Drueke, T.B. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997, 51, 1678–1695. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, A.H.; Van Craenenbroeck, E.M.; Van Ackeren, K.; Hoymans, V.Y.; Verpooten, G.A.; Vrints, C.J.; Couttenye, M.M. Impaired vascular function contributes to exercise intolerance in chronic kidney disease. Nephrol. Dial Transpl. 2016, 31, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K. Principles of exercise testing and Interpretation. J. Cardiopulm. Rehabil. Prev. 1987, 7, 189. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Lewis, N.P.; Saunders, M.J.; Cochlin, D.L.; Davies, M.E.; Hutton, R.D.; Fox, K.A.; Coles, G.A.; Williams, J.D. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet 1990, 335, 489–493. [Google Scholar] [CrossRef]
- Robertson, H.T.; Haley, N.R.; Guthrie, M.; Cardenas, D.; Eschbach, J.W.; Adamson, J.W. Recombinant erythropoietin improves exercise capacity in anemic hemodialysis patients. Am. J. Kidney Dis. 1990, 15, 325–332. [Google Scholar] [CrossRef]
- Bundschu, H.D.; Pfeilsticker, D.; Matthews, C.; Ritz, E. Myopathy in experimental uremia. Res. Exp. Med. 1975, 165, 205–212. [Google Scholar] [CrossRef]
- Moore, G.E.; Parsons, D.B.; Stray-Gundersen, J.; Painter, P.L.; Brinker, K.R.; Mitchell, J.H. Uremic myopathy limits aerobic capacity in hemodialysis patients. Am. J. Kidney Dis. 1993, 22, 277–287. [Google Scholar] [CrossRef]
- Campistol, J.M. Uremic myopathy. Kidney Int. 2002, 62, 1901–1913. [Google Scholar] [CrossRef]
- Cooke, G.A.; Marshall, P.; al-Timman, J.K.; Wright, D.J.; Riley, R.; Hainsworth, R.; Tan, L.B. Physiological cardiac reserve: Development of a non-invasive method and first estimates in man. Heart 1998, 79, 289–294. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Beauvais, F.; Tan, L.B. Peak exercise responses in heart failure: Back to basics. Eur. Heart J. 2009, 30, 2962–2964. [Google Scholar] [CrossRef] [PubMed]
- Fellstrom, B.; Holdaas, H.; Jardine, A. Functional cardiopulmonary exercise testing in potential renal transplant recipients. J. Am. Soc. Nephrol. 2014, 25, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Tabet, J.Y.; Logeart, D.; Bourgoin, P.; Tokmakova, M.; Dahan, M. A non-invasively determined surrogate of cardiac power (‘circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur. Heart J. 2002, 23, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.E.; Guazzi, M.; Myers, J.; Chase, P.; Bensimhon, D.; Cahalin, L.P.; Peberdy, M.A.; Ashley, E.; West, E.; Daniels, K.M.; et al. Ventilatory power: A novel index that enhances prognostic assessment of patients with heart failure. Circ. Heart Fail. 2012, 5, 621–626. [Google Scholar] [CrossRef]
- Shephard, R.J. Tests of maximum oxygen intake. A critical review. Sports Med. 1984, 1, 99–124. [Google Scholar] [CrossRef]




| Parameter | Formula |
|---|---|
| VO2 (L/min) | CO × C(a-v)O2 |
| C(a-v)O2 (mL/dL) | VO2/CO |
| O2 pulse (mL/beat) | VO2/HR |
| Estimated SV [14] (mL/beat) | |
| MAP (mmHg) | MAP = DBP + 0.412 (SBP − DBP) |
| CPO (W) | CO × MAP × 2.22 × 10−3 |
| Peak Circulatory Power (mmHg L min−1) | MAP × VO2peak |
| Peak Ventilatory Power (mmHg) | |
| Ventilatory efficiency slope | VE/VCO2 |
| eLBM | (0.407 × Weight) + (0.267 × Height) − 19.2 |
| CKD (n = 70) | HF (n = 25) | p-Value | |
|---|---|---|---|
| Age (year) | 48.4 ± 12.6 | 49.4 ± 14.6 | NS |
| BMI (kg/m2) | 27.8 ± 3.9 | 25.1 ± 3.2 | <0.05 |
| Hb (g/dL) | 13.3 ± 1.8 | 14.4 ± 1.1 | <0.05 |
| eGFR (mL/min) | 33.9 ± 23.5 | 69.3 ± 16.9 | <0.05 |
| Peak RER | 1.16 ± 0.09 | 1.10 ± 0.29 | NS |
| VO2peak (L/min) | 2.66 ± 0.57 | 1.61 ± 0.37 | <0.05 |
| AT (L/min) | 1.81 ± 0.47 | 1.11 ± 0.35 | <0.05 |
| AT (mL/min/kg) | 21.23 ± 5.18 | 14.61 ± 4.37 | <0.05 |
| Peak CO (L/min) | 19.7 ± 2.6 | 12.5 ± 2.4 | <0.05 |
| Peak C(a-v)O2 (mL/dL) | 13.4 ± 1.9 | 12.9 ± 2.2 | NS |
| Peak HR (beats/min) | 153.4 ± 19.9 | 126.9 ± 30.8 | <0.05 |
| Peak SV (mL/beat) | 129.9 ± 20.7 | 105.1 ± 37.0 | <0.05 |
| Peak O2 pulse (mL/beat) | 17.48 ± 3.62 | 13.35 ± 4.54 | <0.05 |
| Peak Circ pwr (mmHg L min−1) | 277.9 ± 68.4 | 135.9 ± 47.9 | <0.05 |
| Ventilatory Power | 5.36 ± 1.13 | 3.20 ± 1.21 | <0.05 |
| Peak CPO (W) | 4.54 ± 0.77 | 2.34 ± 0.63 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnappa, S.; Shih, M.-C.; Tu, Y.-K.; Mooney, A. The Interpretation of Standard Cardiopulmonary Exercise Test Indices of Cardiac Function in Chronic Kidney Disease. J. Clin. Med. 2023, 12, 7456. https://doi.org/10.3390/jcm12237456
Chinnappa S, Shih M-C, Tu Y-K, Mooney A. The Interpretation of Standard Cardiopulmonary Exercise Test Indices of Cardiac Function in Chronic Kidney Disease. Journal of Clinical Medicine. 2023; 12(23):7456. https://doi.org/10.3390/jcm12237456
Chicago/Turabian StyleChinnappa, Shanmugakumar, Ming-Chieh Shih, Yu-Kang Tu, and Andrew Mooney. 2023. "The Interpretation of Standard Cardiopulmonary Exercise Test Indices of Cardiac Function in Chronic Kidney Disease" Journal of Clinical Medicine 12, no. 23: 7456. https://doi.org/10.3390/jcm12237456