Parkinson’s Disease Non-Motor Subtypes Classification in a Group of Slovenian Patients: Actuarial vs. Data-Driven Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Assessments
2.2. NMS Subtyping
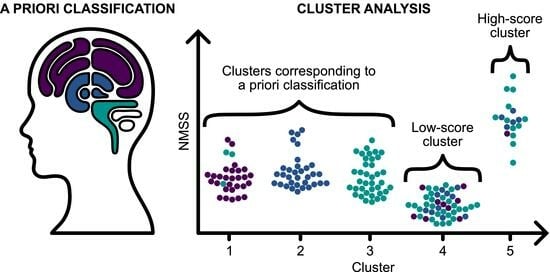
2.2.1. A Priori Classification Approach
- Cortical subtype; the sum of the NMSS symptom scores in the domains of cognitive impairment and apathy (NMSS items 7, 8, 16, 17, 18) is higher than the sum of the scores in the symptom domains classified as limbic and brainstem.
- Limbic subtype; the sum of the NMSS symptom scores in the depression, anxiety, pain, and fatigue domains (NMSS items 4, 9, 10, 11, 12, 27, 29) is higher than the sum of the scores in the symptom domains classified as cortical and brainstem.
- Brainstem subtype; the sum of the NMSS symptom scores in the domain of brainstem symptoms (NMSS items 1, 2, 3, 5, 19, 20, 21, 22, 23, 24, 25, 26) is higher than the sum of the scores in the symptom domains classified as cortical and limbic.
2.2.2. NMS Subtyping Based on K-Means Clustering
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. A Priori Classification Approach
3.3. NMS Subtypes Resulting from k-Means Clustering
3.4. Relationship between NMS Subtyping Based on a Priori Approach and k-Means Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marinus, J.; Zhu, K.; Marras, C.; Aarsland, D.; van Hilten, J.J. Risk Factors for Non-Motor Symptoms in Parkinson’s Disease. Lancet Neurol. 2018, 17, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M. Nonmotor Symptoms in Parkinson’s Disease. Int. J. Neurosci. 2011, 121, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Koh, S.-B. Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease. JMD 2015, 8, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the Development of Parkinson’s Disease-Related Pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- van Uem, J.M.T.; Marinus, J.; Canning, C.; van Lummel, R.; Dodel, R.; Liepelt-Scarfone, I.; Berg, D.; Morris, M.E.; Maetzler, W. Health-Related Quality of Life in Patients with Parkinson’s Disease—A Systematic Review Based on the ICF Model. Neurosci. Biobehav. Rev. 2016, 61, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Marras, C. Subtypes of Parkinson’s Disease: State of the Field and Future Directions. Curr. Opin. Neurol. 2015, 28, 382–386. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Postuma, R.B. Subtypes of Parkinson’s Disease: What Do They Tell Us About Disease Progression? Curr. Neurol. Neurosci. Rep. 2017, 17, 34. [Google Scholar] [CrossRef]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Parkinsonism and Related Disorders Non Motor Subtypes and Parkinson’ s Disease. Park. Relat. Disord. 2016, 22, S41–S46. [Google Scholar] [CrossRef]
- Dujardin, K.; Langlois, C.; Plomhause, L.; Carette, A.-S.; Delliaux, M.; Duhamel, A.; Defebvre, L. Apathy in Untreated Early-Stage Parkinson Disease: Relationship with Other Non-Motor Symptoms. Mov. Disord. 2014, 29, 1796–1801. [Google Scholar] [CrossRef]
- Brown, R.G.; Landau, S.; Hindle, J.V.; Playfer, J.; Samuel, M.; Wilson, K.C.; Hurt, C.S.; Anderson, R.J.; Carnell, J.; Dickinson, L.; et al. Depression and Anxiety Related Subtypes in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2011, 82, 803–809. [Google Scholar] [CrossRef]
- Romenets, S.R.; Gagnon, J.-F.; Latreille, V.; Panniset, M.; Chouinard, S.; Montplaisir, J.; Postuma, R.B. Rapid Eye Movement Sleep Behavior Disorder and Subtypes of Parkinson’s Disease. Mov. Disord. 2012, 27, 996–1003. [Google Scholar] [CrossRef]
- Marras, C.; Chaudhuri, K.R. Nonmotor Features of Parkinson’s Disease Subtypes. Mov. Disord. 2016, 31, 1095–1102. [Google Scholar] [CrossRef]
- Qian, E.; Huang, Y. Subtyping of Parkinson’s Disease—Where Are We up to? Aging Dis. 2019, 10, 1130–1139. [Google Scholar] [CrossRef]
- Jankovic, J.; McDermott, M.; Carter, J.; Gauthier, S.; Goetz, C.; Golbe, L.; Huber, S.; Koller, W.; Olanow, C.; Shoulson, I. Variable Expression of Parkinson’s Disease: A Base-Line Analysis of the DATATOP Cohort. The Parkinson Study Group. Neurology 1990, 40, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. Unified Parkinson’s Disease Rating Scale. Recent. Dev. Park. Dis. 1987, 153–163. [Google Scholar]
- Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to Identify Tremor Dominant and Postural Instability/Gait Difficulty Groups with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale: Comparison with the Unified Parkinson’s Disease Rating Scale. Mov. Disord. 2013, 28, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Hendricks, R.M.; Khasawneh, M.T. A Systematic Review of Parkinson’s Disease Cluster Analysis Research. Aging Dis. 2021, 12, 1567–1586. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-C. A Short Survey on Data Clustering Algorithms. In Proceedings of the 2015 Second International Conference on Soft Computing and Machine Intelligence (ISCMI), Hong Kong, China, 23–24 November 2015; pp. 64–68. [Google Scholar]
- Edmonds, E.C.; Smirnov, D.S.; Thomas, K.R.; Graves, L.V.; Bangen, K.J.; Delano-Wood, L.; Galasko, D.R.; Salmon, D.P.; Bondi, M.W. Data-Driven vs. Consensus Diagnosis of MCI: Enhanced Sensitivity for Detection of Clinical, Biomarker, and Neuropathologic Outcomes. Neurology 2021, 97, e1288–e1299. [Google Scholar] [CrossRef]
- Kenney, L.E.; Ratajska, A.M.; Lopez, F.V.; Price, C.C.; Armstrong, M.J.; Bowers, D. Mapping Actuarial Criteria for Parkinson’s Disease-Mild Cognitive Impairment onto Data-Driven Cognitive Phenotypes. Brain Sci. 2021, 12, 54. [Google Scholar] [CrossRef]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H.; Reidel, B.W.; Bush, A.J. Epidemiology of Insomnia, Depression, and Anxiety. Sleep 2005, 28, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Brobert, G.P.; Johansson, S.; Jick, S.S.; Meier, C.R. Risk of Incident Depression in Patients with Parkinson Disease in the UK. Eur. J. Neurol. 2011, 18, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Minkova, L.; Habich, A.; Peter, J.; Kaller, C.P.; Eickhoff, S.B.; Klöppel, S. Gray Matter Asymmetries in Aging and Neurodegeneration: A Review and Meta-Analysis. Hum. Brain Mapp. 2017, 38, 5890–5904. [Google Scholar] [CrossRef] [PubMed]
- Cubo, E.; Martínez-Martín, P.; González-Bernal, J.; Casas, E.; Arnaiz, S.; Miranda, J.; Gámez, P.; Santos-García, D.; Adarmes, A.D.; Almeria, M.; et al. Effects of Motor Symptom Laterality on Clinical Manifestations and Quality of Life in Parkinson’s Disease. J. Park. Dis. 2020, 10, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Todorova, A.; Jenner, P.; Ray Chaudhuri, K. Non-Motor Parkinson’s: Integral to Motor Parkinson’s, yet Often Neglected. Pract. Neurol. 2014, 14, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathology of Sporadic Parkinson’s Disease: Evaluation and Changes of Concepts. Mov. Disord. 2011, 27, 8–30. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Lue, L.; Sue, L.I.; Bachalakuri, J.; Henry-Watson, J.; Sasse, J.; Boyer, S.; Shirohi, S.; Brooks, R.; et al. Unified Staging System for Lewy Body Disorders: Correlation with Nigrostriatal Degeneration, Cognitive Impairment and Motor Dysfunction. Acta Neuropathol. 2009, 117, 613–634. [Google Scholar] [CrossRef]
- Halliday, G.; Lees, A.; Stern, M. Milestones in Parkinson’s Disease—Clinical and Pathologic Features. Mov. Disord. 2011, 26, 1015–1021. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson's Disease: A Clinico-Pathological Study of 100 Cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS Research Criteria for Prodromal Parkinson’s Disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; MacPhee, G.; MacMahon, D.; et al. The Metric Properties of a Novel Non-Motor Symptoms Scale for Parkinson’s Disease: Results from an International Pilot Study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Hoops, S.; Nazem, S.; Siderowf, A.D.; Duda, J.E.; Xie, S.X.; Stern, M.B.; Weintraub, D. Validity of the MoCA and MMSE in the Detection of MCI and Dementia in Parkinson Disease. Neurology 2009, 73, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, N.N.W.; Sellbach, A.; Matheson, S.; Marsh, R.; Silburn, P.A.; O’Sullivan, J.D.; Byrne, G.J.; Mellick, G.D. Validity of Hamilton Depression Inventory in Parkinson’s Disease. Mov. Disord. 2007, 22, 399–403. [Google Scholar] [CrossRef]
- Leentjens, A.F.G.; Dujardin, K.; Marsh, L.; Richard, I.H.; Starkstein, S.E.; Martinez-Martin, P. Anxiety Rating Scales in Parkinson’s Disease: A Validation Study of the Hamilton Anxiety Rating Scale, the Beck Anxiety Inventory, and the Hospital Anxiety and Depression Scale. Mov. Disord. 2011, 26, 407–415. [Google Scholar] [CrossRef]
- Kurtis, M.M.; Balestrino, R.; Rodriguez-Blazquez, C.; Forjaz, M.J.; Martinez-Martin, P. A Review of Scales to Evaluate Sleep Disturbances in Movement Disorders. Front. Neurol. 2018, 9, 369. [Google Scholar] [CrossRef]
- Pedersen, K.F.; Alves, G.; Larsen, J.P.; Tysnes, O.-B.; Møller, S.G.; Brønnick, K. Psychometric Properties of the Starkstein Apathy Scale in Patients With Early Untreated Parkinson Disease. Am. J. Geriatr. Psychiatry 2012, 20, 142–148. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic Review of Levodopa Dose Equivalency Reporting in Parkinson’s Disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Reijnders, J.S.A.M.; Ehrt, U.; Lousberg, R.; Aarsland, D.; Leentjens, A.F.G. The Association between Motor Subtypes and Psychopathology in Parkinson’s Disease. Park. Relat. Disord. 2009, 15, 379–382. [Google Scholar] [CrossRef]
- Zarei, M.; Ibarretxe-Bilbao, N.; Compta, Y.; Hough, M.; Junque, C.; Bargallo, N.; Tolosa, E.; Martí, M.J. Cortical Thinning Is Associated with Disease Stages and Dementia in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2013, 84, 875–882. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Evans, J.R.; Goris, A.; Foltynie, T.; Ban, M.; Robbins, T.W.; Brayne, C.; Kolachana, B.S.; Weinberger, D.R.; Sawcer, S.J.; et al. The Distinct Cognitive Syndromes of Parkinson’s Disease: 5 Year Follow-up of the CamPaIGN Cohort. Brain 2009, 132, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Vacca, J.L.; Massman, P.J.; Liao, T.Y. The Influence of Handedness on the Clinical Presentation and Neuropsychology of Alzheimer Disease. Arch. Neurol. 1999, 56, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- de Leon, M.J.; la Regina, M.E.; Ferris, S.H.; Gentes, C.I.; Miller, J.D. Reduced Incidence of Left-Handedness in Clinically Diagnosed Dementia of the Alzheimer Type. Neurobiol. Aging 1986, 7, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, A.; Ng, M.; Al Omran, Y.; Alfaro-Almagro, F.; McCarthy, P.; Marchini, J.; Bennett, D.L.; Smith, S.; Douaud, G.; Furniss, D. Handedness, Language Areas and Neuropsychiatric Diseases: Insights from Brain Imaging and Genetics. Brain 2019, 142, 2938–2947. [Google Scholar] [CrossRef]
- Jellinger, K.A. Very Old Onset Parkinsonism: A Clinical-Pathological Study. Park. Relat. Disord. 2018, 57, 39–43. [Google Scholar] [CrossRef]
- Kostic, V.; Przedborski, S.; Flaster, E.; Sternic, N. Early Development of Levodopa-induced Dyskinesias and Response Fluctuations in Young-onset Parkinson’s Disease. Neurology 1991, 41, 202. [Google Scholar] [CrossRef]
- Paolo, B.; Angelo, A.; Carlo, C.; Roberto, M.; Letterio, M.; Avarello, T.P.; Eugenio, B.; Antonino, C.; Gabriella, C.; Roberto, C.; et al. The PRIAMO Study: A Multicenter Assessment of Nonmotor Symptoms and Their Impact on Quality of Life in Parkinson’s Disease. Mov. Disord. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Oppo, V.; Melis, M.; Melis, M.; Tomassini Barbarossa, I.; Cossu, G. “Smelling and Tasting” Parkinson’s Disease: Using Senses to Improve the Knowledge of the Disease. Front. Aging Neurosci. 2020, 12, 43. [Google Scholar] [CrossRef]
- Vennemann, M.M.; Hummel, T.; Berger, K. The Association between Smoking and Smell and Taste Impairment in the General Population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef]
- Da Ré, A.F.; Gurgel, L.G.; Buffon, G.; Moura, W.E.R.; Marques Vidor, D.C.G.; Maahs, M.A.P. Tobacco Influence on Taste and Smell: Systematic Review of the Literature. Int. Arch. Otorhinolaryngol. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Sharer, J.D.; Leon-Sarmiento, F.E.; Morley, J.F.; Weintraub, D.; Doty, R.L. Olfactory Dysfunction in Parkinson’s Disease: Positive Effect of Cigarette Smoking. Mov. Disord. 2015, 30, 859–862. [Google Scholar] [CrossRef]
- Cong, S.; Xiang, C.; Zhang, S.; Zhang, T.; Wang, H.; Cong, S. Prevalence and Clinical Aspects of Depression in Parkinson’s Disease: A Systematic Review and Meta-analysis of 129 Studies. Neurosci. Biobehav. Rev. 2022, 141, 104749. [Google Scholar] [CrossRef]
- Baba, T.; Kikuchi, A.; Hirayama, K.; Nishio, Y.; Hosokai, Y.; Kanno, S.; Hasegawa, T.; Sugeno, N.; Konno, M.; Suzuki, K.; et al. Severe Olfactory Dysfunction Is a Prodromal Symptom of Dementia Associated with Parkinson’s Disease: A 3 Year Longitudinal Study. Brain 2012, 135, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Amboni, M.; Erro, R.; Longo, K.; Vitale, C.; Moccia, M.; Pierro, A.; Santangelo, G.; De Rosa, A.; De Michele, G.; et al. Gender Differences in Non-Motor Symptoms in Early, Drug Naïve Parkinson’s Disease. J. Neurol. 2013, 260, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, D.; Thurman, D.J.; Gwinn-Hardy, K.; Mohamed, M.; Chaudhuri, A.R.; Zalutsky, R. How Common Are the “Common” Neurologic Disorders? Neurology 2007, 68, 326–337. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Q.; B Joshi, R.; Liu, Y.; Akbar, M.; Song, B.-J.; Zhou, S.; Wang, X. Role of Alcohol Drinking in Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2316. [Google Scholar] [CrossRef]
- Daviet, R.; Aydogan, G.; Jagannathan, K.; Spilka, N.; Koellinger, P.D.; Kranzler, H.R.; Nave, G.; Wetherill, R.R. Associations between Alcohol Consumption and Gray and White Matter Volumes in the UK Biobank. Nat. Commun. 2022, 13, 1175. [Google Scholar] [CrossRef]
- Lowery-Gionta, E.G.; Marcinkiewcz, C.A.; Kash, T.L. Functional Alterations in the Dorsal Raphe Nucleus Following Acute and Chronic Ethanol Exposure. Neuropsychopharmacology 2015, 40, 590–600. [Google Scholar] [CrossRef]
- Ma, C.; Pavlova, M.; Li, J.; Liu, Y.; Sun, Y.; Huang, Z.; Wu, S.; Gao, X. Alcohol Consumption and Probable Rapid Eye Movement Sleep Behavior Disorder. Ann. Clin. Transl. Neurol. 2018, 5, 1176–1183. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.-F. Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms. Front. Neurosci. 2020, 14, 602697. [Google Scholar] [CrossRef]
- Chen, J.Q.A.; Scheltens, P.; Groot, C.; Ossenkoppele, R. Associations Between Caffeine Consumption, Cognitive Decline, and Dementia: A Systematic Review. J. Alzheimers Dis. 2020, 78, 1519–1546. [Google Scholar] [CrossRef]
- Cho, B.-H.; Choi, S.-M.; Kim, B.C. Gender-Dependent Effect of Coffee Consumption on Tremor Severity in de Novo Parkinson’s Disease. BMC Neurol. 2019, 19, 194. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Wang, H.X. Smoking and Parkinson’s and Alzheimer’s Disease: Review of the Epidemiological Studies. Behav. Brain Res. 2000, 113, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ng, S.Y.-E.; Chia, N.S.-Y.; Setiawan, F.; Tay, K.-Y.; Au, W.-L.; Tan, E.-K.; Tan, L.C.-S. Non-Motor Symptoms in Early Parkinson’s Disease with Different Motor Subtypes and Their Associations with Quality of Life. Eur. J. Neurol. 2019, 26, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Pont-Sunyer, C.; Hotter, A.; Gaig, C.; Seppi, K.; Compta, Y.; Katzenschlager, R.; Mas, N.; Hofeneder, D.; Brücke, T.; Bayés, A.; et al. The Onset of Nonmotor Symptoms in Parkinson’s Disease (the Onset Pd Study). Mov. Disord. 2015, 30, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Durcan, R.; Wiblin, L.; Lawson, R.A.; Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Brooks, D.J.; Pavese, N.; Burn, D.J.; ICICLE-PD Study Group. Prevalence and Duration of Non-Motor Symptoms in Prodromal Parkinson’s Disease. Eur. J. Neurol. 2019, 26, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Ba, F.; Obaid, M.; Wieler, M.; Camicioli, R.; Martin, W.R.W. Parkinson Disease: The Relationship Between Non-Motor Symptoms and Motor Phenotype. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2016, 43, 261–267. [Google Scholar] [CrossRef]



| Overall (n = 168) (100%) | Cortical (n = 38) (22.6%) | Limbic (n = 48) (28.6%) | Brainstem (n = 82) (48.8%) | p-Value | Adj. p-Value * | Post Hoc Statistical Analysis ** | |
|---|---|---|---|---|---|---|---|
| Gender (male) | 100 (59.9%) | 24 (63.2%) | 20 (41.7%) | 56 (68.3%) | 0.010 χ2 | 0.020 | Limbic-Male/Femae |
| Age (years) | 71.70 ± 9.57 | 77.08 ± 8.42 | 68.81 ± 8.88 | 70.89 ± 9.57 | <0.001 KW | 0.055 (sig.) | Cortical-Limbic Cortical-Brainstem |
| Age at onset (years) | 65.45 ± 10.18 | 72.21 ± 8.06 | 61.90 ± 9.55 | 64.40 ± 10.06 | <0.001 KW | 0.014 | Cortical-Limbic Cortical-Brainstem |
| Education ≥ 3. stage based on ISCED (%) | 123 (73.2%) | 18 (47.3%) | 39 (81.2%) | 66 (80.4%) | <0.001 KW | 0.028 | Cortical-Limbic Cortical-Brainstem |
| Right-handed (%) | 144 (85.7%) | 23 (60.5%) | 45 (93.8%) | 76 (92.7%) | 0.001 χ2 | 0.018 | Cortical-Left/Right |
| Disease duration > 10 years (%) | 34 (20.2%) | 3 (7.9%) | 12 (25.0%) | 19 (23.2%) | 0.045 KW | 0.077 | Cortical-Limbic |
| Family history | 27 (16.1%) | 11 (28.9%) | 6 (12.5%) | 10 (12.2%) | 0.235 χ2 | 0.294 | |
| Side of onset—right (%) | 92 (54.8%) | 25 (65.8%) | 23 (47.9%) | 44 (53.7%) | 0.245 χ2 | 0.299 | |
| Motor subtype | |||||||
| TD (%) | 87 (51.8%) | 2 (5.3%) | 36 (75.0%) | 49 (59.8%) | <0.001 χ2 | 0.004 | Cortical–TD/PIGD Limbic–TD/PIGD) Brainstem-PIGD |
| PIGD (%) | 61 (36.3%) | 33 (86.8%) | 7 (14.6%) | 21 (25.6%) | |||
| Intermediate (%) | 20 (11.9%) | 3 (7.9%) | 5 (10.4%) | 12 (14.6%) | |||
| No. of prodromes | 2.2 ± 1.34 | 2.16 ± 1.26 | 2.17 ± 1.04 | 2.24 ± 1.53 | 0.947 KW | 0.947 | |
| No. of NMS | 6.88 ± 3.21 | 5.89 ± 2.15 | 7.25 ± 2.99 | 7.12 ± 3.66 | 0.154 KW | 0.207 | |
| MoCA | 25.77 ± 2.55 | 23.97 ± 1.94 | 26.50 ± 2.54 | 26.18 ± 2.43 | <0.001 KW | 0.005 | Cortical-Limbic Cortical-Brainstem |
| HAM-A | 6.05 ± 5.32 | 2.58 ± 1.73 | 10.90 ± 5.07 | 4.83 ± 4.57 | <0.001 KW | 0.005 | All pairs |
| HAM-D | 6.95 ± 5.61 | 3.11 ± 1.69 | 12.54 ± 5.50 | 5.46 ± 4.28 | <0.001 KW | 0.005 | All pairs |
| UPDRS III | 37.37 ± 10.96 | 35.76 ± 9.11 | 39.85 ± 10.51 | 36.66 ± 11.85 | 0.130 KW | 0.183 | |
| H&Y | 2.45 ± 0.70 | 2.82 ± 0.51 | 2.33 ± 0.75 | 2.35 ± 0.69 | <0.001 KW | 0.004 | Cortical-Limbic Cortical-Brainstem |
| LED | 725.00 ± 285.76 | 733.68 ± 283.27 | 685.62 ± 276.14 | 744.02 ± 293.50 | 0.522 KW | 0.563 | |
| ESS | 6.72 ± 4.24 | 6.79 ± 4.69 | 5.10 ± 2.68 | 7.63 ± 4.53 | 0.002 KW | 0.006 | Limbic-Brainstem |
| FSS | 31.81 ± 13.18 | 29.76 ± 10.74 | 35.67 ± 11.65 | 30.50 ± 14.64 | 0.023 KW | 0.044 | Cortical-Limbic |
| RBDSQ | 4.92 ± 2.65 | 4.39 ± 2.52 | 4.23 ± 2.15 | 5.57 ± 2.83 | 0.008 KW | 0.018 | Limbic-Brainstem |
| SAS | 11.35 ± 6.39 | 14.63 ± 5.75 | 10.35 ± 5.29 | 10.40 ± 6.81 | 0.001 KW | 0.003 | Cortical-Limbic Cortical-Brainstem |
| NMSS | 59.38 ± 36.94 | 48.92 ± 21.43 | 66.58 ± 34.87 | 60.00 ± 42.61 | 0.077 KW | 0.128 | |
| Cluster 1 (n = 37) (22.0%) | Cluster 2 (n = 35) (20.8%) | Cluster 3 (n = 38) (22.6%) | Cluster 4 (n = 46) (27.4%) | Cluster 5 (n = 12) (7.1%) | p-Value | Adj. p-Value * | |
|---|---|---|---|---|---|---|---|
| Gender (male) (%) | 21 (67.7%) | 11 (33.3%) | 21 (61.8%) | 34 (65.3%) | 13 (72.2%) | 0.015 χ2 | 0.025 |
| Age at onset (years) | 72.74 ± 8.71 | 62.42 ± 9.09 | 64.91 ± 10.05 | 64.10 ± 9.77 | 63.39 ± 11.03 | <0.001 KW | 0.019 |
| Disease duration > 10 years (%) | 4 (12.9%) | 7 (21.2%) | 10 (29.4%) | 5 (9.6%) | 8 (44.4%) | 0.003 KW | 0.006 |
| Side of onset—right (%) | 20 (64.5%) | 18 (54.5%) | 18 (52.9%) | 29 (55.8%) | 7 (38.9%) | 0.543 χ2 | 0.543 |
| Motor subtype | |||||||
| TD (%) | 2 (6.5%) | 28 (84.8%) | 19 (55.9%) | 29 (55.8%) | 9 (50.0%) | <0.001 χ2 | 0.003 |
| PIGD (%) | 28 (90.3%) | 5 (15.2%) | 9 (26.5%) | 12 (23.1%) | 7 (38.9%) | ||
| Intermediate (%) | 1 (3.2%) | 0 (0.0%) | 6 (17.6%) | 11 (21.2%) | 2 (11.1%) | ||
| No. of NMS | 6.58 ± 1.65 | 7.58 ± 2.54 | 8.03 ± 2.67 | 4.35 ± 2.66 | 11.28 ± 2.35 | <0.001 KW | 0.005 (sig.) |
| MoCA | 23.45 ± 1.75 | 26.55 ± 2.21 | 25.94 ± 2.41 | 27.10 ± 2.12 | 24.22 ± 2.32 | <0.001 KW | 0.004 |
| HAM-A | 2.87 ± 2.13 | 12.33 ± 4.76 | 4.15 ± 2.23 | 3.02 ± 2.43 | 12.39 ± 5.17 | <0.001 KW | 0.004 |
| HAM-D | 3.06 ± 1.18 | 14.27 ± 4.89 | 4.88 ± 2.27 | 3.83 ± 1.92 | 163.17 ± 5.44 | <0.001 KW | 0.003 |
| UPDRS III | 36.29 ± 7.26 | 39.21 ± 6.80 | 38.85 ± 9.56 | 32.63 ± 11.93 | 46.72 ± 14.90 | <0.001 KW | 0.003 |
| H&Y | 2.90 ± 0.40 | 2.27 ± 0.63 | 2.50 ± 0.66 | 2.04 ± 0.52 | 3.11 ± 0.83 | <0.001 KW | 0.003 |
| ESS | 7.13 ± 4.77 | 5.18 ± 2.69 | 8.29 ± 5.24 | 5.52 ± 3.19 | 9.33 ± 4.23 | <0.001 KW | 0.003 |
| FSS | 31.42 ± 9.91 | 35.12 ± 10.68 | 34.56 ± 14.14 | 24.77 ± 12.77 | 41.56 ± 12.35 | <0.001 KW | 0.002 |
| RBDSQ | 4.90 ± 2.71 | 4.48 ± 2.32 | 5.85 ± 2.80 | 4.10 ± 2.07 | 6.39 ± 3.33 | 0.010 KW | 0.018 |
| SAS | 15.71 ± 5.62 | 10.85 ± 5.69 | 11.24 ± 5.56 | 7.77 ± 5.07 | 15.28 ± 7.51 | <0.001 KW | 0.002 |
| NMSS | 56.90 ± 15.91 | 68.27 ± 21.48 | 64.47 ± 22.61 | 25.54 ± 13.04 | 135.44 ± 27.20 | <0.001 KW | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrijan, T.; Zmazek, J.; Menih, M. Parkinson’s Disease Non-Motor Subtypes Classification in a Group of Slovenian Patients: Actuarial vs. Data-Driven Approach. J. Clin. Med. 2023, 12, 7434. https://doi.org/10.3390/jcm12237434
Petrijan T, Zmazek J, Menih M. Parkinson’s Disease Non-Motor Subtypes Classification in a Group of Slovenian Patients: Actuarial vs. Data-Driven Approach. Journal of Clinical Medicine. 2023; 12(23):7434. https://doi.org/10.3390/jcm12237434
Chicago/Turabian StylePetrijan, Timotej, Jan Zmazek, and Marija Menih. 2023. "Parkinson’s Disease Non-Motor Subtypes Classification in a Group of Slovenian Patients: Actuarial vs. Data-Driven Approach" Journal of Clinical Medicine 12, no. 23: 7434. https://doi.org/10.3390/jcm12237434