Periostin Is a Biomarker of Rheumatoid Arthritis-Associated Interstitial Lung Disease
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Subjects
2.2. Study Protocol
2.3. Measurement of Periostin by ELISA
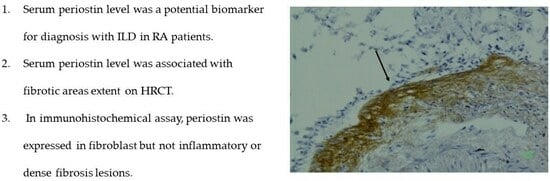
2.4. Immunohistochemical Assay
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Concordance of HRCT Evaluation between Two Investigators
3.2. ROC Analysis to Differentiate RA-ILD Patients from RA Patients without ILD or Healthy Controls
3.3. Correlation between Serum Biomarker Levels and Clinical Data of RA-ILD Patients
3.4. Immunohistochemical Assay Using Anti-Periostin Monoclonal Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, S.K.; Song, Y.J.; Kang, J.; Jeong, S.A.; Kim, H.W.; Choi, C.B.; Kim, T.H.; Jun, J.B.; Bae, S.C.; et al. Clinical characteristics of rheumatoid arthritis patients with interstitial lung disease: Baseline data of a single-center prospective cohort. Arthritis Res. Ther. 2023, 25, 43. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Di Carlo, M.; Tardella, M.; Giovagnoni, A. High-resolution computed tomography of the lung in patients with rheumatoid arthritis: Prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine 2019, 98, e17088. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Inoue, E.E.; Tanaka, G.; Singh, E.; Sato, D.; Hoshi, K.; Shidara, M.; Hara, S.; Momohara, A.; Taniguchi, N.; et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand. J. Rheumatol. 2010, 39, 360–367. [Google Scholar] [CrossRef]
- Bongartz, T.; Nannini, C.; Medina-Velasquez, Y.F.; Achenbach, S.J.; Crowson, C.S.; Ryu, J.H.; Vassallo, R.; Gabriel, S.E.; Matteson, E.L. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2010, 62, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Koduri, G.; Norton, S.; Young, A.; Cox, N.; Davies, P.; Devlin, J.; Dixey, J.; Gough, A.; Prouse, P.; Winfield, J.; et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: Results from an inception cohort. Rheumatology 2010, 49, 1483–1489. [Google Scholar] [CrossRef]
- Kondoh, Y.; Makino, S.; Ogura, T.; Suda, T.; Tomioka, H.; Amano, H.; Anraku, M.; Enomoto, N.; Fujii, T.; Fujisawa, T.; et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir. Investig. 2021, 59, 709–740. [Google Scholar] [CrossRef]
- Assayag, D.; Lee, J.S.; King, T.E., Jr. Rheumatoid arthritis associated interstitial lung disease: A review. Medicina 2014, 74, 158–165. [Google Scholar]
- Hozumi, H.; Nakamura, Y.; Johkoh, T.; Sumikawa, H.; Colby, T.V.; Kono, M.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; Inui, N.; et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: A retrospective case control study. BMJ Open 2013, 3, e003132. [Google Scholar] [CrossRef]
- Dawson, J.K.; Fewins, H.E.; Desmond, J.; Lynch, M.P.; Graham, D.R. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 517–521. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.S.; Park, I.N.; Jang, S.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of fibrotic interstitial pneumonia: Idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Adegunsoye, A.; Oldham, J.M.; Bellam, S.K.; Montner, S.; Churpek, M.M.; Noth, I.; Vij, R.; Strek, M.E.; Chung, J.H. Computed tomography honeycombing identifies a progressive fibrotic phenotype with increased mortality across diverse interstitial lung diseases. Ann. Am. Thorac. Soc. 2019, 16, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Lee, H.K.; Lee, C.K.; Chae, E.J.; Jang, S.J.; Colby, T.V.; Kim, D.S. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc. Diffus. Lung Dis. 2013, 30, 103–112. [Google Scholar]
- Yamakawa, H.; Sato, S.; Nishizawa, T.; Kawabe, R.; Oba, T.; Kato, A.; Horikoshi, M.; Akasaka, K.; Amano, M.; Sasaki, H.; et al. Impact of radiological honeycombing in rheumatoid arthritis-associated interstitial lung disease. BMC Pulm. Med. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Cassone, G.; Manfredi, A.; Vacchi, C.; Luppi, F.; Coppi, F.; Salvarani, C.; Sebastiani, M. Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease: Lights and Shadows. J. Clin. Med. 2020, 9, 1082. [Google Scholar] [CrossRef]
- Yokoyama, A.; Kondo, K.; Nakajima, M.; Matsushima, T.; Takahashi, T.; Nishimura, M.; Bando, M.; Sugiyama, Y.; Totani, Y.; Ishizaki, T.; et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006, 11, 164–168. [Google Scholar] [CrossRef]
- Takahashi, H.; Fujishima, T.; Koba, H.; Murakami, S.; Kurokawa, K.; Shibuya, Y.; Shiratori, M.; Kuroki, Y.; Abeet, S. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am. J. Respir. Crit. Care Med. 2000, 162, 1109–1114. [Google Scholar] [CrossRef]
- Ishikawa, N.; Hattori, N.; Yokoyama, A.; Kohno, N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir. Investig. 2012, 50, 3–13. [Google Scholar] [CrossRef]
- Oyama, T.; Kohno, N.; Yokoyama, A.; Hirasawa, Y.; Hiwada, K.; Oyama, H.; Okuda, Y.; Takasugi, K. Detection of interstitial pneumonitis in patients with rheumatoid arthritis by measuring circulating levels of KL-6, a human MUC1 mucin. Lung 1997, 175, 379–385. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, E.Y.; Ha, Y.J.; Kang, E.H.; Lee, Y.J.; Song, Y.W. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 2019, 21, 58. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.C.; Lee, B.Y.; Lee, C.K.; Kim, M.Y.; Jang, S.J.; Lee, H.S.; Moon, J.; Colby, T.V.; Kim, D.S. The Value of Biomarkers as Predictors of Outcome in Patients with Rheumatoid Arthritis-Associated Usual Interstitial Pneumonia. Sarcoidosis Vasc. Diffuse Lung Dis. 2016, 33, 216–223. [Google Scholar] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Hotokebuchi, T.; Izuhara, K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Izuhara, K.; Ohta, S.; Ono, J.; Hoshino, T. Ability of Periostin as a New Biomarker of Idiopathic Pulmonary Fibrosis. Adv. Exp. Med. Biol. 2019, 1132, 79–87. [Google Scholar] [PubMed]
- Okamoto, M.; Hoshino, T.; Kitasato, Y.; Sakazaki, Y.; Kawayama, T.; Fujimoto, K.; Ohshima, K.; Shiraishi, H.; Uchida, M.; Ono, J.; et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur. Respir. J. 2011, 37, 1119–1127. [Google Scholar] [CrossRef]
- Naik, P.K.; Bozyk, P.D.; Bentley, J.K.; Popova, A.P.; Birch, C.M.; Wilke, C.A.; Fry, C.D.; White, E.S.; Sisson, T.H.; Tayob, N.; et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L1046–L1056. [Google Scholar] [CrossRef]
- Ohta, S.; Okamoto, M.; Fujimoto, K.; Sakamoto, N.; Takahashi, K.; Yamamoto, H.; Kushima, H.; Ishii, H.; Akasaka, K.; Ono, J.; et al. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS ONE 2017, 12, e0174547. [Google Scholar] [CrossRef]
- Nukui, Y.; Miyazaki, Y.; Masuo, M.; Okamoto, T.; Furusawa, H.; Tateishi, T.; Kishino, M.; Tateishi, U.; Ono, J.; Ohta, S.; et al. Periostin as a predictor of prognosis in chronic bird-related hypersensitivity pneumonitis. Allergol. Int. 2019, 68, 363–369. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E., Jr.; Lasky, J.A.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kuroki, Y.; Tanaka, H.; Saito, T.; Kurokawa, K.; Chiba, H.; Sagawa, A.; Nagae, H.; Abe, S. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am. J. Respir. Crit. Care Med. 2000, 162, 258–263. [Google Scholar] [CrossRef]
- Asano, Y.; Ihn, H.; Yamane, K.; Yazawa, N.; Kubo, M.; Fujimoto, M.; Tamaki, K. Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2001, 44, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Kerschan-Schindl, K.; Ebenbichler, G.; Föeger-Samwald, U.; Leiss, H.; Gesslbauer, C.; Herceg, M.; Stummvoll, G.; Marculescu, R.; Crevenna, R.; Pietschmann, P. Rheumatoid arthritis in remission: Decreased myostatin and increased serum levels of periostin. Wien. Klin. Wochenschr. 2019, 131, 1–7. [Google Scholar] [CrossRef]
- Brown, J.M.; Mantoku, A.; Sabokbar, A.; Oppermann, U.; Hassan, A.B.; Kudo, A.; Athanasou, N. Periostin expression in neoplastic and non-neoplastic diseases of bone and joint. Clin. Sarcoma Res. 2018, 5, 18. [Google Scholar] [CrossRef]
- Shimizu, H.; Sakamoto, S.; Okamoto, M.; Isshiki, T.; Ono, J.; Shimizu, S.; Hoshino, T.; Izuhara, K.; Homma, S. Association of serum monomeric periostin level with outcomes of acute exacerbation of idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Ann. Transl. Med. 2021, 9, 739. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. INBUILD Trial Investigators. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Yamakawa, H.; Sato, S.; Tsumiyama, E.; Nishizawa, T.; Kawabe, R.; Oba, T.; Kamikawa, T.; Horikoshi, M.; Akasaka, K.; Amano, M.; et al. Predictive factors of mortality in rheumatoid arthritis-associated interstitial lung disease analysed by modified HRCT classification of idiopathic pulmonary fibrosis according to the 2018 ATS/ERS/JRS/ALAT criteria. J. Thorac. Dis. 2019, 11, 5247–5257. [Google Scholar] [CrossRef]
- Nurmi, H.; Kaarteenaho, R. The challenges in classifying rheumatoid arthritis-associated interstitial lung disease. J. Thorac. Dis. 2020, 12, 3000–3003. [Google Scholar] [CrossRef]



| p Value | |||||||
|---|---|---|---|---|---|---|---|
| Among | RA-ILD vs. | RA-ILD vs. | RA w/o ILD | ||||
| RA with ILD | RA w/o ILD | Healthy Control | 3 Groups | RA w/o ILD | Control | vs. Control | |
| N | 19 | 20 | 137 | ||||
| Age | 70 (65–76) | 66 (60–73) | 41 (30–50) | <0.0001 * | 0.14 | <0.0001 * | <0.0001 * |
| Male gender | 6 (32%) | 4 (20%) | 91 (66%) | <0.0001 * | 0.48 | 0.0050 * | 0.0001 * |
| Smoker | 6 (32%) | N.D. | 64 (47%) | 0.23 | |||
| LDH (IU/L) | 205.0 (178.0–233.0) | 195.0 (164.3–232.8) | 149.0 (136.0–164.5) | <0.0001 * | 0.66 | <0.0001 * | <0.0001 * |
| SP-D (ng/mL) | 105.0 (50.2–203.0) | 36.1 (22.8–69.5) | 40.5 (26.7–66.3) | 0.0002 * | 0.0012 * | <0.0001 * | 0.72 |
| KL-6 (IU/mL) | 1022.0 (579.0–1467.0) | 214.5 (177.3–273.0) | 274.0 (226.5–336.0) | <0.0001 * | <0.0001 * | <0.0001 * | 0.0048 * |
| Monomeric periostin (pg/mL) | 15.9 (14.3–20.1) | 13.4 (10.8–16.3) | 8.3 (7.2–9.9) | <0.0001 * | 0.0043 * | <0.0001 * | <0.0001 * |
| Total periostin (pg/mL) | 100.0 (77.0–140.0) | 73.5 (55.8–84.8) | 63.0 (52.5–75.5) | <0.0001 * | 0.0035 * | <0.0001 * | 0.10 |
| Pulmonary Function | |
| Vital capacity (%) | 84.4 (66.7–107.3) |
| DLCO (%) | 71.4 (60.7–86.1) |
| HRCT pattern | |
| Definite UIP | 5 (25%) |
| Possible UIP | 3 (15%) |
| Inconsistent with UIP | 12 (60%) |
| Extent of abnormal area on chest HRCT (%) | |
| Normal lung area | 137.5 (100.0–155.0) |
| Honeycombing | 0 (0–7.5) |
| Reticulation | 25.0 (10.0–40.0) |
| Ground-glass attenuation | 22.5 (20.0–47.5) |
| Airspace consolidation | 0 (0–10.0) |
| Fibrosis score | 25.0 (10.0–40.0) |
| Inflammation score | 32.5 (20.0–50.0) |
| TBE grade on HRCT | 13.0 (7.0–30.0) |
| AUC | Standard Error | p Value | Cut-Off Value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| Distinguishing RA without ILD patients | ||||||
| LDH (IU/L) | 0.459 | 0.0028 | 0.53 | 218.0 | 73.7 | 35.0 |
| KL-6 (IU/mL) | 0.939 | 0.0020 | <0.0001 * | 329.0 | 100.0 | 80.0 |
| SP-D (ng/mL) | 0.803 | 0.0088 | 0.0002 * | 93.9 | 63.2 | 90.0 |
| Monomeric periostin (ng/mL) | 0.767 | 0.081 | 0.0089 * | 14.3 | 79.0 | 75.0 |
| Total periostin (ng/mL) | 0.767 | 0.013 | 0.0027 * | 98.0 | 63.2 | 85.0 |
| Distinguishing Healthy controls | ||||||
| LDH (IU/L) | 0.886 | 0.0096 | <0.0001 * | 177.0 | 84.2 | 88.3 |
| KL-6 (IU/mL) | 0.972 | 0.0032 | <0.0001 * | 423.0 | 89.5 | 95.6 |
| SP-D (ng/mL) | 0.794 | 0.0050 | <0.0001 * | 79.5 | 68.4 | 87.6 |
| Monomeric periostin (ng/mL) | 0.994 | 0.443 | <0.0001 * | 12.5 | 100.0 | 96.3 |
| Total periostin (ng/mL) | 0.876 | 0.0126 | <0.0001 * | 75.0 | 84.2 | 74.0 |
| Monomeric Periostin (ng/mL) | Total Periostin (ng/mL) | KL-6 (IU/mL) | SP-D (ng/mL) | LDH (IU/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | p Value | R | p Value | R | p Value | R | p Value | R | p Value | |
| Age (years) | 0.17 | 0.50 | 0.26 | 0.28 | −0.069 | 0.78 | −0.0053 | 0.98 | 0.19 | 0.44 |
| Serum biomarkers | ||||||||||
| SP-D (ng/mL) | 0.073 | 0.77 | 0.12 | 0.61 | 0.53 | 0.019 * | - | - | 0.24 | 0.33 |
| LDH(IU/L) | −0.30 | 0.22 | 0.078 | 0.75 | 0.36 | 0.13 | 0.24 | 0.33 | - | - |
| KL-6 (IU/mL) | 0.23 | 0.35 | 0.29 | 0.23 | - | - | 0.53 | 0.019 * | 0.36 | 0.13 |
| Pulmonary function | ||||||||||
| Vital capacity (%) | −0.32 | 0.19 | −0.53 | 0.022 * | −0.063 | 0.80 | −0.35 | 0.16 | −0.21 | 0.39 |
| DLCO (%) | −0.18 | 0.52 | −0.38 | 0.16 | −0.50 | 0.060 | −0.45 | 0.090 | −0.31 | 0.27 |
| Extent of abnormal area on chest HRCT (%) | ||||||||||
| Normal lung area | −0.57 | 0.011 * | −0.62 | 0.0045 * | −0.44 | 0.062 | −0.35 | 0.15 | −0.13 | 0.58 |
| Honeycombing | 0.54 | 0.018 * | 0.65 | 0.0025 * | 0.28 | 0.25 | −0.12 | 0.64 | −0.12 | 0.64 |
| Reticulation | 0.51 | 0.013 * | 0.78 | 0.0002 * | 0.18 | 0.47 | 0.045 | 0.85 | 0.18 | 0.47 |
| GGA | −0.031 | 0.90 | −0.14 | 0.56 | 0.44 | 0.061 | 0.54 | 0.017 * | 0.25 | 0.31 |
| Airspace consolidation | 0.19 | 0.44 | 0.069 | 0.78 | −0.47 | 0.041 * | −0.26 | 0.29 | −0.41 | 0.081 |
| Fibrosis score | 0.55 | 0.014 * | 0.78 | <0.0001 * | 0.18 | 0.47 | 0.051 | 0.83 | 0.11 | 0.65 |
| Inflammation score | −0.091 | 0.71 | −0.13 | 0.59 | 0.25 | 0.31 | 0.31 | 0.19 | 0.14 | 0.56 |
| TBE grade on HRCT | 0.47 | 0.041 * | 0.70 | 0.0008 * | 0.33 | 0.16 | 0.41 | 0.079 | 0.19 | 0.42 |
| Definite UIP Pattern | p Value | ||
|---|---|---|---|
| Yes | No | ||
| N | 5 | 14 | |
| LDH (IU/L) | 200.0 (163.0–218.0) | 207.5 (180.3–262.8) | 0.19 |
| KL-6 (IU/mL) | 1414.0 (554.5–1905.0) | 942.5 (541.8–1367.3) | 0.52 |
| SP-D (ng/mL) | 93.9 (35.9–240.0) | 123.0 (56.7–212.0) | 0.52 |
| Monomeric periostin (pg/mL) | 20.1 (18.6–50.5) | 15.3 (13.5–17.5) | 0.018 * |
| Total periostin (pg/mL) | 143.0(121.9–202.5) | 90.0 (73.8–115.0) | 0.0095 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matama, G.; Okamoto, M.; Fujimoto, K.; Johkoh, T.; Tominaga, M.; Mukae, H.; Sakamoto, N.; Komiya, K.; Umeki, K.; Komatsu, M.; et al. Periostin Is a Biomarker of Rheumatoid Arthritis-Associated Interstitial Lung Disease. J. Clin. Med. 2023, 12, 7100. https://doi.org/10.3390/jcm12227100
Matama G, Okamoto M, Fujimoto K, Johkoh T, Tominaga M, Mukae H, Sakamoto N, Komiya K, Umeki K, Komatsu M, et al. Periostin Is a Biomarker of Rheumatoid Arthritis-Associated Interstitial Lung Disease. Journal of Clinical Medicine. 2023; 12(22):7100. https://doi.org/10.3390/jcm12227100
Chicago/Turabian StyleMatama, Goushi, Masaki Okamoto, Kiminori Fujimoto, Takeshi Johkoh, Masaki Tominaga, Hiroshi Mukae, Noriho Sakamoto, Kosaku Komiya, Kenji Umeki, Masamichi Komatsu, and et al. 2023. "Periostin Is a Biomarker of Rheumatoid Arthritis-Associated Interstitial Lung Disease" Journal of Clinical Medicine 12, no. 22: 7100. https://doi.org/10.3390/jcm12227100