Effects of Thrombin-Based Hemostatic Agent in Total Knee Arthroplasty: Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis and Statistical Methods
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Risk of Bias Assessment
3.4. Outcomes for Meta-Analysis
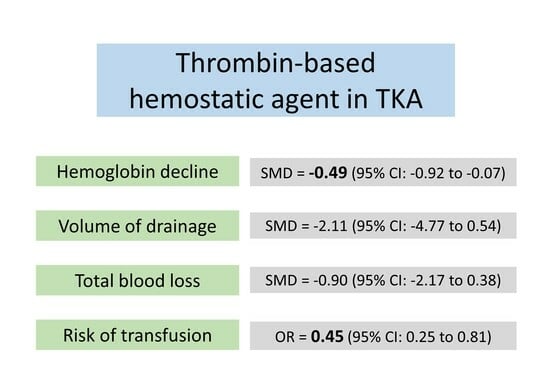
3.4.1. Hemoglobin Decline
3.4.2. Volume of Drainage
3.4.3. Total Blood Loss
3.4.4. Risk of Allogenic Transfusion
3.5. Publication Bias
3.6. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goh, G.S.; Fillingham, Y.A.; Ong, C.B.; Krueger, C.A.; Courtney, P.M.; Hozack, W.J. Redefining Indications for Modern Cementless Total Knee Arthroplasty: Clinical Outcomes and Survivorship in Patients >75 Years Old. J. Arthroplast. 2022, 37, 476–481.e1. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Q.; Wei, B.G.; Zhang, X.S.; Torsha, T.T.; Xiao, J.; Shi, Z.J. Blood loss of total knee arthroplasty in osteoarthritis: An analysis of influential factors. J. Orthop. Surg. Res. 2018, 13, 325. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Huang, X.; Liu, R. Tranexamic Acid Reduces Occult Blood Loss, Blood Transfusion, and Improves Recovery of Knee Function after Total Knee Arthroplasty: A Comparative Study. J. Knee Surg. 2018, 31, 239–246. [Google Scholar] [CrossRef]
- Klika, A.K.; Small, T.J.; Saleh, A.; Szubski, C.R.; Chandran Pillai, A.L.; Barsoum, W.K. Primary total knee arthroplasty allogenic transfusion trends, length of stay, and complications: Nationwide inpatient sample 2000-2009. J. Arthroplast. 2014, 29, 2070–2077. [Google Scholar] [CrossRef]
- Suh, Y.S.; Choi, H.S.; Lee, J.S.; Jang, B.W.; Hwang, J.; Song, M.G.; Joo, J.; Chung, H.; Lee, J.J.; Nho, J.H. Transfusion Trends of Knee Arthroplasty in Korea: A Nationwide Study Using the Korean National Health Insurance Service Sample Data. Int. J. Environ. Res. Public Health 2022, 19, 5982. [Google Scholar] [CrossRef]
- Liu, D.; Dan, M.; Martinez Martos, S.; Beller, E. Blood Management Strategies in Total Knee Arthroplasty. Knee Surg. Relat. Res. 2016, 28, 179–187. [Google Scholar] [CrossRef]
- Echave, M.; Oyaguez, I.; Casado, M.A. Use of Floseal(R), a human gelatine-thrombin matrix sealant, in surgery: A systematic review. BMC Surg. 2014, 14, 111. [Google Scholar] [CrossRef]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilico, S.; Bindi, F.; et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 68. [Google Scholar] [CrossRef]
- Sae-Jung, S.; Apiwatanakul, P. Chitosan Pad, Cellulose Membrane, or Gelatin Sponge for Peridural Bleeding: An Efficacy Study on a Lumbar Laminectomized Rat Model. Asian Spine J. 2018, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.A.; Neveleff, D.J. Achieving hemostasis with topical hemostats: Making clinically and economically appropriate decisions in the surgical and trauma settings. AORN J. 2011, 94, S1–S20. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, R.; Albrech, M.; Bauer, G. Use of FloSeal hemostatic matrix in a patient with severe postpartum hemorrhage. J. Obstet. Gynaecol. Res. 2012, 38, 435–437. [Google Scholar] [CrossRef]
- Nasso, G.; Piancone, F.; Bonifazi, R.; Romano, V.; Visicchio, G.; De Filippo, C.M.; Impiombato, B.; Fiore, F.; Bartolomucci, F.; Alessandrini, F.; et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann. Thorac. Surg. 2009, 88, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Yoshida, M.; Okada, M.; Nakamura, Y.; Yawatari, K.; Nakayama, E. Effectiveness of a Gelatin-Thrombin Matrix Sealant (Floseal(R)) for Reducing Blood Loss During Microendoscopic Decompression Surgery for Lumbar Spinal Canal Stenosis: A Retrospective Cohort Study. Glob. Spine J. 2021, 13, 764–770. [Google Scholar] [CrossRef]
- Waldert, M.; Remzi, M.; Klatte, T.; Klingler, H.C. FloSeal reduces the incidence of lymphoceles after lymphadenectomies in laparoscopic and robot-assisted extraperitoneal radical prostatectomy. J. Endourol. 2011, 25, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Keenan, J.; Mason, J.; Hseih, J.T.; Batstone, M. Prospective study examining the use of thrombin-gelatin matrix (Floseal) to prevent post dental extraction haemorrhage in patients with inherited bleeding disorders. Int. J. Oral. Maxillofac. Surg. 2022, 51, 426–430. [Google Scholar] [CrossRef]
- Bonduelle, Q.; Biggs, T.C.; Sipaul, F. Floseal: A novel application technique for the treatment of challenging epistaxis. Clin. Otolaryngol. 2020, 45, 960–962. [Google Scholar] [CrossRef]
- Brand, Y.; Narayanan, V.; Prepageran, N.; Waran, V. A Cost-Effective Delivery System for FloSeal During Endoscopic and Microscopic Brain Surgery. World Neurosurg. 2016, 90, 492–495. [Google Scholar] [CrossRef]
- Gazzeri, R.; Galarza, M.; Alfier, A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg. Technol. Int. 2012, 22, 49–54. [Google Scholar]
- Ujam, A.; Awad, Z.; Wong, G.; Tatla, T.; Farrell, R. Safety trial of Floseal((R)) haemostatic agent in head and neck surgery. Ann. R. Coll. Surg. Engl. 2012, 94, 336–339. [Google Scholar] [CrossRef]
- Bae, K.C.; Cho, C.H.; Lee, K.J.; Son, E.S.; Lee, S.W.; Lee, S.J.; Lim, K.H. Efficacy of intra-articular injection of thrombin-based hemostatic agent in the control of bleeding after primary total knee arthroplasty. Knee Surg. Relat. Res. 2014, 26, 236–240. [Google Scholar] [CrossRef]
- Comadoll, J.L.; Comadoll, S.; Hutchcraft, A.; Krishnan, S.; Farrell, K.; Kreuwel, H.T.; Bechter, M. Comparison of hemostatic matrix and standard hemostasis in patients undergoing primary TKA. Orthopedics 2012, 35, e785–e793. [Google Scholar] [CrossRef]
- Di Francesco, A.; Flamini, S.; Fiori, F.; Mastri, F. Hemostatic matrix effects on blood loss after total knee arthroplasty: A randomized controlled trial. Indian. J. Orthop. 2013, 47, 474–481. [Google Scholar] [CrossRef]
- Helito, C.P.; Bonadio, M.B.; Sobrado, M.F.; Giglio, P.N.; Pecora, J.R.; Camanho, G.L.; Demange, M.K. Comparison of Floseal(R) and Tranexamic Acid for Bleeding Control after Total Knee Arthroplasty: A Prospective Randomized Study. Clinics 2019, 74, e1186. [Google Scholar] [CrossRef]
- Helito, C.P.; Gobbi, R.G.; Castrillon, L.M.; Hinkel, B.B.; Pecora, J.R.; Camanho, G.L. Comparison of Floseal(r) and electrocautery in hemostasis after total knee arthroplasty. Acta Ortop. Bras. 2013, 21, 320–322. [Google Scholar] [CrossRef]
- Kim, H.J.; Fraser, M.R.; Kahn, B.; Lyman, S.; Figgie, M.P. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: A randomized controlled trial. J. Bone Jt. Surg. Am. 2012, 94, 1160–1165. [Google Scholar] [CrossRef]
- Suarez, J.C.; Slotkin, E.M.; Alvarez, A.M.; Szubski, C.R.; Barsoum, W.K.; Patel, P.D. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J. Arthroplast. 2014, 29, 1950–1955. [Google Scholar] [CrossRef]
- Fu, X.; Tian, P.; Xu, G.J.; Sun, X.L.; Ma, X.L. Thrombin-Based Hemostatic Agent in Primary Total Knee Arthroplasty. J. Knee Surg. 2017, 30, 121–127. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Zhang, T.; Ma, J.X.; Jiang, X.; Wang, Y.; Ma, X.L. The efficacy of a thrombin-based hemostatic agent in primary total knee arthroplasty: A meta-analysis. J. Orthop. Surg. Res. 2014, 9, 90. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; John & Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Shim, S.R.; Kim, S.J. Intervention meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019008. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Velyvis, J.H. Gelatin matrix use reduces postoperative bleeding after total knee arthroplasty. Orthopedics 2015, 38, e118–e123. [Google Scholar] [CrossRef]
- Yen, S.H.; Lin, P.C.; Wu, C.T.; Wang, J.W. Comparison of Effects of a Thrombin-Based Hemostatic Agent and Topical Tranexamic Acid on Blood Loss in Patients with Preexisting Thromboembolic Risk Undergoing a Minimally Invasive Total Knee Arthroplasty. A Prospective Randomized Controlled Trial. Biomed. Res. Int. 2021, 2021, 2549521. [Google Scholar] [CrossRef]
- Schwab, P.E.; Thienpont, E. Use of a haemostatic matrix does not reduce blood loss in minimally invasive total knee arthroplasty. Blood Transfus. 2015, 13, 435–441. [Google Scholar] [CrossRef]
- Schwab, P.E.; Thienpont, E. Use of a haemostatic matrix (Floseal(R)) does not reduce blood loss in minimally invasive total knee arthroplasty performed under continued aspirin. Blood Transfus. 2016, 14, 134–139. [Google Scholar] [CrossRef]






| Author | Year | Number (F/C) | Age (Years) (F/C) | Male (F/C) | BMI (kg/m2) (F/C) | Antithrombotic Agent | Transfusion Criteria (g/dL) | Dosage (mL) |
|---|---|---|---|---|---|---|---|---|
| Kim HJ [27] | 2012 | 97/99 | 72.7/70.1 | N/S | N/S | Aspirin or warfarin | N/S | 10 |
| Helito CP [26] | 2013 | 10/10 | 67.8/66.6 | N/S | N/S | Enoxaparin | V/S change a | 10 |
| Di Francesco A [24] | 2013 | 51/42 | 67.9/70.2 | 24/17 | 26.0/26.2 | Enoxaparin | Hb 8.5 | 10 |
| Suarez JC [28] | 2014 | 56/52 | 65.9/65.1 | 20/21 | 29.8/33.7 | Enoxaparin | Hb 8.0 | 5 |
| Bae KC [22] | 2014 | 50/50 | 68.8/69.0 | 4/8 | 26.4/24.8 | N/S | Hb 8.5 | 10 |
| Velyvis JH [34] | 2015 | 157/100 | 72.5/73.0 | 71/47 | N/S | N/S | Hb 8 or 9 and associated symptoms b | 10 or 5 |
| Helito CP [25] | 2019 | 30/30 | N/S | N/S | N/S | Enoxaparin | N/S | 10 |
| Yen SH [31] | 2021 | 34/35 | 69.7/69.7 | 6/3 | 29.4/28.6 | Enoxaparin | N/S | 10 |
| Author | Year | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|
| Kim HJ [27] | 2012 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Helito CP [26] | 2013 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Di Francesco A [24] | 2013 | Some concerns | Low risk | Low risk | Some concerns | Low risk | Some concerns |
| Suarez JC [28] | 2014 | Low risk | Low risk | Low risk | Low risk | Some concerns | Some concerns |
| Bae KC [22] | 2014 | Some concerns | Low risk | Low risk | Low risk | Some concerns | Some concerns |
| Velyvis JH [34] | 2015 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Helito CP [25] | 2019 | Some concerns | Low risk | Low risk | Some concerns | Some concerns | Some concerns |
| Yen SH [35] | 2021 | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Kim, T.W.; Chang, C.B.; Han, M.; Go, J.J.; Park, B.K.; Jo, W.-L.; Lee, Y.-K. Effects of Thrombin-Based Hemostatic Agent in Total Knee Arthroplasty: Meta-Analysis. J. Clin. Med. 2023, 12, 6656. https://doi.org/10.3390/jcm12206656
Park J-W, Kim TW, Chang CB, Han M, Go JJ, Park BK, Jo W-L, Lee Y-K. Effects of Thrombin-Based Hemostatic Agent in Total Knee Arthroplasty: Meta-Analysis. Journal of Clinical Medicine. 2023; 12(20):6656. https://doi.org/10.3390/jcm12206656
Chicago/Turabian StylePark, Jung-Wee, Tae Woo Kim, Chong Bum Chang, Minji Han, Jong Jin Go, Byung Kyu Park, Woo-Lam Jo, and Young-Kyun Lee. 2023. "Effects of Thrombin-Based Hemostatic Agent in Total Knee Arthroplasty: Meta-Analysis" Journal of Clinical Medicine 12, no. 20: 6656. https://doi.org/10.3390/jcm12206656