Reproductive Outcomes of Women with Turner Syndrome Undergoing Oocyte Vitrification: A Retrospective Multicenter Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Outcome Measure
2.4. Statistical Analysis
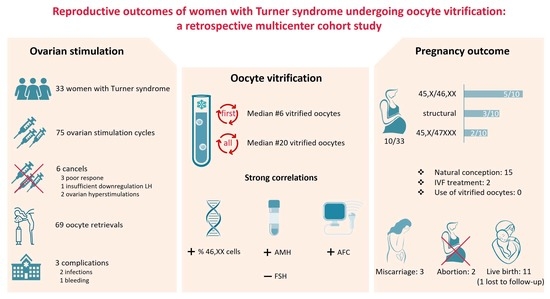
3. Results
3.1. Baseline Characteristics
3.2. Outcome of Ovarian Stimulation and Vitrification of Oocytes
3.3. Pregnancy Outcomes
4. Discussion
4.1. Interpretation of the Findings
4.2. Considerations for Clinical Practice
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stochholm, K.; Juul, S.; Juel, K.; Naeraa, R.W.; Gravholt, C.H. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.N.; Sane, S.; Bhartiya, D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol. Hum. Reprod. 2003, 9, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, K.; Cortvrindt, R.; Verlinde, F.; De Schepper, J.; Bourgain, C.; Smitz, J. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil. Steril. 2004, 81, 1112–1119. [Google Scholar] [CrossRef]
- Fitz, V.W.; Law, J.R.; Peavey, M. Karyotype is associated with timing of ovarian failure in women with Turner syndrome. J. Pediatr. Endocrinol. Metab. 2021, 34, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Calanchini, M.; Aye, C.Y.L.; Orchard, E.; Baker, K.; Child, T.; Fabbri, A.; Mackillop, L.; Turner, H.E. Fertility issues and pregnancy outcomes in Turner syndrome. Fertil. Steril. 2020, 114, 144–154. [Google Scholar] [CrossRef]
- Bernard, V.; Donadille, B.; Zenaty, D.; Courtillot, C.; Salenave, S.; Brac de la Perrière, A.; Albarel, F.; Fèvre, A.; Kerlan, V.; Brue, T.; et al. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum. Reprod. 2016, 31, 782–788. [Google Scholar] [CrossRef]
- Oktay, K.; Bedoschi, G.; Berkowitz, K.; Bronson, R.; Kashani, B.; McGovern, P.; Pal, L.; Quinn, G.; Rubin, K. Fertility Preservation in Women with Turner Syndrome: A Comprehensive Review and Practical Guidelines. J. Pediatr. Adolesc. Gynecol. 2016, 29, 409–416. [Google Scholar] [CrossRef]
- Sutton, E.J.; McInerney-Leo, A.; Bondy, C.A.; Gollust, S.E.; King, D.; Biesecker, B. Turner syndrome: Four challenges across the lifespan. Am. J. Med. Genet. A 2005, 139, 57–66. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rubin, K.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017, 177, G1–G70. [Google Scholar] [CrossRef]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Schleedoorn, M.J.; van der Velden, A.; Braat, D.D.M.; Peek, R.; Fleischer, K. To Freeze or Not to Freeze? An Update on Fertility Preservation In Females with Turner Syndrome. Pediatr. Endocrinol. Rev. 2019, 16, 369–382. [Google Scholar]
- Pasquino, A.M.; Passeri, F.; Pucarelli, I.; Segni, M.; Municchi, G. Spontaneous pubertal development in Turner’s syndrome. Italian Study Group for Turner’s Syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1810–1813. [Google Scholar]
- Brouillet, S.; Ranisavljevic, N.; Sonigo, C.; Haquet, E.; Bringer-Deutsch, S.; Loup-Cabaniols, V.; Hamamah, S.; Willems, M.; Anahory, T. Should we perform oocyte accumulation to preserve fertility in women with Turner syndrome? A multicenter study and systematic review of the literature. Hum. Reprod. 2023, 38, 1733–1745. [Google Scholar] [CrossRef]
- Talaulikar, V.S.; Conway, G.S.; Pimblett, A.; Davies, M.C. Outcome of ovarian stimulation for oocyte cryopreservation in women with Turner syndrome. Fertil. Steril. 2019, 111, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Martel, R.A.; Blakemore, J.K.; Fino, M.E. The use of oocyte cryopreservation for fertility preservation in patients with sex chromosome disorders: A case series describing outcomes. J. Assist. Reprod. Genet. 2022, 39, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Strypstein, L.; Van Moer, E.; Nekkebroeck, J.; Segers, I.; Tournaye, H.; Demeestere, I.; Dolmans, M.M.; Verpoest, W.; De Vos, M. First live birth after fertility preservation using vitrification of oocytes in a woman with mosaic Turner syndrome. J. Assist. Reprod. Genet. 2022, 39, 543–549. [Google Scholar] [CrossRef]
- Viuff, M.; Gravholt, C.H. Turner Syndrome and Fertility. Ann. d’Endocrinol. 2022, 83, 244–249. [Google Scholar] [CrossRef]
- Specchia, C.; Baggiani, A.; Immediata, V.; Ronchetti, C.; Cesana, A.; Smeraldi, A.; Scaravelli, G.; Levi-Setti, P.E. Oocyte Cryopreservation in Oncological Patients: Eighteen Years Experience of a Tertiary Care Referral Center. Front. Endocrinol. 2019, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; García-Velasco, J.A.; Coello, A.; Domingo, J.; Pellicer, A.; Remohí, J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil. Steril. 2016, 105, 755–764.e758. [Google Scholar] [CrossRef] [PubMed]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef]
- Peek, R.; Nadesapillai, S.; Thi Nguyen, T.Y.; Vassart, S.; Smeets, D.; van de Zande, G.; Camboni, A.; Braat, D.; van der Velden, J.; Donnez, J.; et al. Assessment of folliculogenesis in ovarian tissue from young patients with Turner syndrome using a murine xenograft model. Fertil. Steril. 2023, 120, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Klein, B.M.; Arce, J.C. Comparison of antimüllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil. Steril. 2015, 103, 923–930.e1. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, H.; Hu, S.; Liu, S.; Li, F.; Jin, L. Efficacy of three COS protocols and predictability of AMH and AFC in women with discordant ovarian reserve markers: A retrospective study on 19,239 patients. J. Ovarian Res. 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.P.; Fischer, M.B.; Mola, G.; Mikkelsen, T.B.; Cleemann, L.H.; Gravholt, C.H.; Viuff, M.H.; Juul, A.; Pedersen, A.T.; Main, K.M. AMH and other markers of ovarian function in patients with Turner syndrome—A single center experience of transition from pediatric to gynecological follow up. Front. Endocrinol. 2023, 14, 1173600. [Google Scholar] [CrossRef] [PubMed]
- Lunding, S.A.; Aksglaede, L.; Anderson, R.A.; Main, K.M.; Juul, A.; Hagen, C.P.; Pedersen, A.T. AMH as Predictor of Premature Ovarian Insufficiency: A Longitudinal Study of 120 Turner Syndrome Patients. J. Clin. Endocrinol. Metab. 2015, 100, E1030–E1038. [Google Scholar] [CrossRef] [PubMed]
- Biniasch, M.; Laubender, R.P.; Hund, M.; Buck, K.; Geyter, C.D. Intra- and inter-cycle variability of anti-Müllerian hormone (AMH) levels in healthy women during non-consecutive menstrual cycles: The BICYCLE study. Clin. Chem. Lab. Med. (CCLM) 2022, 60, 597–605. [Google Scholar] [CrossRef]
- Hagen, C.P.; Main, K.M.; Kjaergaard, S.; Juul, A. FSH, LH, inhibin B and estradiol levels in Turner syndrome depend on age and karyotype: Longitudinal study of 70 Turner girls with or without spontaneous puberty. Hum. Reprod. 2010, 25, 3134–3141. [Google Scholar] [CrossRef]
- Nadesapillai, S.; van der Velden, J.; van der Coelen, S.; Schleedoorn, M.; Sedney, A.; Spath, M.; Schurink, M.; Oerlemans, A.; IntHout, J.; Beerendonk, I.; et al. TurnerFertility trial: Fertility preservation in young girls with Turner syndrome by freezing ovarian cortex tissue—A prospective intervention study. Fertil. Steril. 2023. [Google Scholar] [CrossRef]
- Peek, R.; Schleedoorn, M.; Smeets, D.; van de Zande, G.; Groenman, F.; Braat, D.; van der Velden, J.; Fleischer, K. Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic 45,X granulosa cells. Hum. Reprod. 2019, 34, 1686–1696. [Google Scholar] [CrossRef]
- Azem, F.; Brener, A.; Malinger, G.; Reches, A.; Many, A.; Yogev, Y.; Lebenthal, Y. Bypassing physiological puberty, a novel procedure of oocyte cryopreservation at age 7: A case report and review of the literature. Fertil. Steril. 2020, 114, 374–378. [Google Scholar] [CrossRef]
- Morgan, T.L.; Kapa, H.M.; Crerand, C.E.; Kremen, J.; Tishelman, A.; Davis, S.; Nahata, L. Fertility counseling and preservation discussions for females with Turner syndrome in pediatric centers: Practice patterns and predictors. Fertil. Steril. 2019, 112, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Luo, K.; Cheng, D.; Xie, P.; Tan, Y.; Hu, L.; Lu, G.; Gong, F.; Lin, G. Reproductive outcomes after preimplantation genetic testing in mosaic Turner syndrome: A retrospective cohort study of 100 cycles. J. Assist. Reprod. Genet. 2021, 38, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.; Meseguer, M.; Mercader, A.; Rubio, C.; Alegre, L.; Vidal, C.; Trabalon, M.; Bosch, E. Preimplantation genetic testing for aneuploidy in patients with partial X monosomy using their own oocytes: Is this a suitable indication? Fertil. Steril. 2020, 114, 346–353. [Google Scholar] [CrossRef] [PubMed]
- van der Coelen, S.; van der Velden, J.; Nadesapillai, S.; Peek, R.; Braat, D.; Schleedoorn, M.; Fleischer, K.; Oerlemans, A. The Decision-Making Process regarding Ovarian Tissue Cryopreservation in Girls with Turner Syndrome by Patients, Parents, and Healthcare Providers: A Mixed-Methods Study. Horm. Res. Paediatr. 2022, 95, 374–383. [Google Scholar] [CrossRef] [PubMed]




| Patient No. | Karyotype Lymphocytes (L)/ Buccal Cells (B) | Age Counseling/ Stimulation (Years) | Comorbidity | Menarche | AFC (n) | FSH (E/L)/ AMH (µg/L) ** | Stimulation Rounds (n) | Total FSH (IE) | Vitrified Oocytes (n) | Pregnancy (n) | Age First Pregnancy (Years) | Children (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L: 45,X (18%)/46,XX (82%) | 16/18 | Cardiac | Yes | 40 | NR/5.10 | 4 | 3845 | 31 | - | - | |
| 2 | L: 45,X (18%)/46,XX (82%) | 16/17 | - | Yes | 48 | 5.10/4.00 | 1 | 2100 | 23 | - | - | |
| 3 | L: 45,X (56%)/46,XX (44%) B: 45,X/46,XX | 17/18 | Thyroid Psychological | Yes | 12 | 5.30/6.70 | 3 | 5250 | 26 | - | - | |
| 4 | L: 45,X (9%)/46,XX (91%) | 17/17 | - | Yes | 10 | 7.40/1.90 | 2 | 1650 | 19 | - | - | |
| 5 | L: 45,X (3%)/46,XX (97%) | 17/18 | Psychological | Yes | 17 | 9.00/2.00 | 3 | 8925 | 20 | - | - | |
| 6 | L: 45,X (27%)/46,XX (73%) B: 45,X (57%)/46,XX (43%) | 18/18 | - | Yes | NR | 2.40/0.35 | 2 | 3150 | 3 | - | - | |
| 7 | L: 45,X (66%)/46,XX (34%) | 18/18 | Pulmonal | Yes | 13 | 3.10/2.80 | 3 | 5625 | 23 | - | - | |
| 8 | L: 45,X (17%)/46,XX (83%) | 18/19 | - | Yes | 16 | 4.00/4.60 | 4 | 7050 | 27 | - | - | |
| 9 | L: 45,X (7%)/46,XX (93%) B: 45,X/46,XX | 19/19 | Renal | Yes | 9 | 5.30/2.40 | 2 | 1800 | 29 | - | - | |
| 10 | L: 45,X (81%)/46,XX (5%)/47,XXX (14%) | 19/20 | Cardiac | Yes * | 1 | 43.23/0.06 | 1 | 5625 | 0 | - | - | |
| 11 | L: 45,X (54%)/46,XX (46%) B: 45,X (65%)/46,XX (35%) | 21/21 | - | Yes | 35 | NR | 3 | 3680 | 38 | - | - | |
| 12 | L: 45,X (17%)/47,XXX (83%) B: 45,X (15%)/46,XX (11%)/47,XXX (74%) | 21/21 | Gastrointestinal | No * | 1 | 17.00/0.03 | 3 | 10,125 | 6 | - | - | |
| 13 | L: 45,X (66%)/46,XX (34%) | 22/22 | Cardiac | Yes | 35 | NR | 2 | 2000 | 6 | Natural conception (3) | NR | 2 |
| 14 | L: 45,X (67%)/47,XXX (33%) B: 45,X (11%)/46,XX (4%)/47,XXX (85%) | 22/22 | - | Yes | 9 | 3.10/1.11 | 3 | 7875 | 20 | - | - | |
| 15 | B: 45,X (9%)/46,XX (91%) | 25/25 | - | Yes | 13 | NR/1.30 | 3 | 6500 | 28 | - | - | |
| 16 | L: 45,X (35%)/46,XX (65%) | 25/26 | Cardiac | Yes | 5 | NR | 2 | 3600 | 8 | IVF (1) | 29 | 1 |
| 17 | L: 45,X (14%)/46,XX (86%) | 26/26 | - | Yes | 11 | 6.70/1.60 | 3 | 6525 | 21 | Natural conception (1) | 30 | 1 |
| 18 | L: 45,X (13%)/46,XX (87%) | 27/28 | Psychological | Yes | 20 | NR/3.10 | 3 | 9450 | 29 | - | - | |
| 19 | L: 45,X (37%)/46,XX (63%) | 29/30 | Cardiac Thryoid | Yes | 16 | NR/8.40 | 3 | 14,700 | 21 | IVF (1) Natural conception (1) | 33 | 2 |
| 20 | L: 45,X (50%)/46,XX (50%) | 29/30 | - | Yes | 15 | NR | 1 | 2475 | 27 | Natural conception (2) | 24 | 1 |
| 21 | L: X-deletion | 17/17 | - | Yes | 3 | 6.90/0.50 | 3 | 9225 | 9 | - | - | |
| 22 | L: 45,X (43%), 46,X, r(X) (57%) | 17/17 | Musculoskeletal | Yes | 12 | NR/2.46 | 2 | 1100 | 26 | - | - | |
| 23 | L: 45,X (75%)/46,X, r(X) (25%) | 17/19 | - | Yes | 0 | 15.00/1.79 | 1 | 3000 | 3 | - | - | |
| 24 | L: X-deletion | 17/17 | - | Yes * | 5 | NR/0.20 | 1 | 2700 | 6 | Natural conception (1) | 20 | NR |
| 25 | L: X-deletion | 17/18 | Cardiac | Yes | 18 | NR/1.00 | 1 | 2100 | 5 | Natural conception (1) | 23 | 0 |
| 26 | L: 45,X (87%)/46,X, r(X) (13%) B: 45,X/46,X, r(X) | 17/18 | - | Yes | 26 | 11.10/0.90 | 2 | 2700 | cancel | - | - | |
| 27 | L: Ring chromosome | 20/21 | - | Yes | 8 | NR/1.06 | 2 | 2925 | 6 | - | - | |
| 28 | L: Isochromosome B: Isochromosome | 25/25 | - | Yes | 14 | 6.40/NR | 3 | 11,100 | 23 | - | - | |
| 29 | L: Isochromosome B: Isochromosome | 30/30 | - | Yes | 11 | 6.10/NR | 3 | 7200 | 24 | Natural conception (2) | 33 | 2 |
| 30 | L: 45,X/47,XXX | 15/16 | Cardiac | Yes | 3 | 2.60/0.14 | 1 | 2475 | 1 | - | ||
| 31 | L: 45,X/47,XXX B: 45,X/47,XXX | 17/17 | - | Yes | 3 | 26.70/NR | 1 | 5400 | 0 | Natural conception (1) | 27 | 1 |
| 32 | L: 45,X (93%)/47,XXX (7%) | 19/19 | - | Yes | NR | 5.90/1.60 | 3 | 6750 | 14 | Natural conception (3) | 22 | 1 |
| 33 | L: 45,X (3%)/47,XXX (97%) | 19/20 | Cardiac Gastrointestinal | Yes * | NR | 20.00/0.02 | 1 | 4725 | cancel | - | - |
| Karyotype Hormone Levels * | 45,X/46,XX (n = 20) | Structural Aberrations (n = 9) | 45,X/47,XXX (n = 4) |
|---|---|---|---|
| n FSH * (E/L), median (IQR) | 12 5.3 (3.3–8.6) | 5 6.9 (6.3–13.0) | 4 13.0 (3.4–25.0) |
| n AMH (µg/L), median (IQR) | 16 2.2 (1.2–4.6) | 7 1.0 (0.5–1.8) | 3 0.1 (Not applicable) |
| n Estradiol (pmol/L), median (IQR) | 11 190 (4–266) | 3 96 (Not applicable) | 2 260 (Not applicable) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadesapillai, S.; Mol, F.; Broer, S.L.; Stevens Brentjens, L.B.P.M.; Verhoeven, M.O.; Heida, K.Y.; Goddijn, M.; van Golde, R.J.T.; Bos, A.M.E.; van der Coelen, S.; et al. Reproductive Outcomes of Women with Turner Syndrome Undergoing Oocyte Vitrification: A Retrospective Multicenter Cohort Study. J. Clin. Med. 2023, 12, 6502. https://doi.org/10.3390/jcm12206502
Nadesapillai S, Mol F, Broer SL, Stevens Brentjens LBPM, Verhoeven MO, Heida KY, Goddijn M, van Golde RJT, Bos AME, van der Coelen S, et al. Reproductive Outcomes of Women with Turner Syndrome Undergoing Oocyte Vitrification: A Retrospective Multicenter Cohort Study. Journal of Clinical Medicine. 2023; 12(20):6502. https://doi.org/10.3390/jcm12206502
Chicago/Turabian StyleNadesapillai, Sapthami, Femke Mol, Simone L. Broer, Linda B. P. M. Stevens Brentjens, Marieke O. Verhoeven, Karst Y. Heida, Mariëtte Goddijn, Ron J. T. van Golde, Annelies M. E. Bos, Sanne van der Coelen, and et al. 2023. "Reproductive Outcomes of Women with Turner Syndrome Undergoing Oocyte Vitrification: A Retrospective Multicenter Cohort Study" Journal of Clinical Medicine 12, no. 20: 6502. https://doi.org/10.3390/jcm12206502