Identifying Patients with Nonalcoholic Fatty Liver Disease in Primary Care: How and for What Benefit?
Abstract
:1. Introduction
2. Pathophysiology of NAFLD
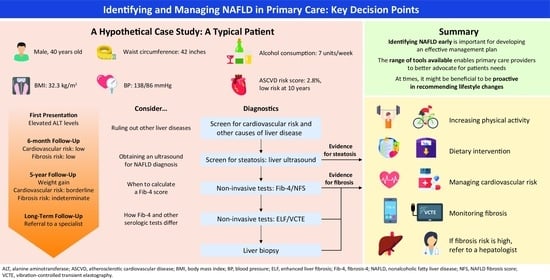
- Age: 40, BMI: 32.3 kg/m2, waist circumference: 42 inches, blood pressure: 138/86 mmHg, and consuming 7 units of alcohol per week;
- Cholesterol: 210 mg/dL, triglyceride: 174 mg/dL, high-density lipoprotein (HDL)-cholesterol: 31 mg/dL, and low-density lipoprotein (LDL)-cholesterol: 144 mg/dL;
- Alanine aminotransferase (ALT): 54 U/L, aspartate aminotransferase (AST): 44 U/L, and platelets: 220 K;
- Hemoglobin A1c (HbA1c): 6.1%;
- He is not taking any medications.
3. When to Pursue a NAFLD Diagnosis
3.1. Pursuing a NAFLD Diagnosis following Abnormalities in Aminotransferases or in Liver Imaging
3.2. Pursuing a NAFLD Diagnosis in High-Risk Patients
4. How to Pursue a Diagnosis
4.1. Conventional US
4.2. CAP Method
4.3. MRI Methods
4.4. Noninvasive Scores
- Previous values: Age: 38, BMI: 27.0 kg/m2, waist circumference: 38 inches, blood pressure: 136/87 mmHg, and consuming 7 units of alcohol per week;
- −
- Current values: Age: 40, BMI: 32.3 kg/m2, waist circumference: 42 inches, blood pressure: 138/86 mmHg, and consuming 7 units of alcohol per week;
- Previous values: Cholesterol: 105 mg/dL, triglyceride: 32 mg/dL, and HDL-cholesterol: 22 mg/dL;
- −
- Current values: Cholesterol: 210 mg/dL, triglyceride: 174 mg/dL, HDL-cholesterol: 31 mg/dL, and LDL-cholesterol: 144 mg/dL;
- Previous values: ALT: 28 U/L and AST: 24 U/L;
- −
- Current values: ALT: 54 U/L, AST: 44 U/L, and platelets: 220 K.
- Atherosclerotic cardiovascular disease (ASCVD) risk score: 2.8%, with a 10-year risk of an ASCVD event;
- Negative viral hepatitis B and C testing;
- Liver ultrasound (US) demonstrating hepatic steatosis;
- Fibrosis-4 (Fib-4): 1.09 (low risk).
5. Interventions: What Are the Options?
5.1. Weight-Loss Interventions
5.2. Cardiovascular Risk Management
- BMI: 29.7 kg/m2, waist circumference: 39.5 inches, blood pressure: 126/78 mmHg, and consuming 3 units of alcohol per week;
- Cholesterol: 190 mg/dL, triglyceride: 158 mg/dL, HDL-cholesterol: 35 mg/dL, and LDL-cholesterol: 123 mg/dL;
- ALT: 32 U/L, AST: 30 U/L, and platelets: 225 K;
- HbA1c: 5.8%;
- Fib-4: 0.94 (low risk);
- ASCVD risk score: 1.6%, with a 10-year risk of an ASCVD event.
- BMI: 37.0 kg/m2, waist circumference: 44 inches, blood pressure: 151/88 mmHg, and consuming 10 units of alcohol per week;
- Cholesterol: 225 mg/dL, triglyceride: 220 mg/dL, HDL-cholesterol: 30 mg/dL, and LDL-cholesterol: 151 mg/dL;
- ALT: 64 U/L, AST: 60 U/L, and platelets: 165 K;
- HbA1c: 6.4%;
- Fib-4: 2.05 (indeterminate risk);
- ASCVD risk: 6.2%, with 10-year risk of an ASCVD event.
5.3. Fibrosis Risk Assessments
Noninvasive Scores for Fibrosis
5.4. Referral to a Hepatologist
- VCTE: 9.9 kPa (high risk).
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Dai, Y.N.; Wang, Y.M.; Zhou, Q.Y.; Yu, C.H.; Li, Y.M. Prevalence of nonalcoholic fatty liver disease and economy. Dig. Dis. Sci. 2015, 60, 3194–3202. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 global NAFLD prevalence: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef]
- Global Burden of Disease Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Noureddin, M.; Vipani, A.; Bresee, C.; Todo, T.; Kim, I.K.; Alkhouri, N.; Setiawan, V.W.; Tran, T.; Ayoub, W.S.; Lu, S.C.; et al. NASH leading cause of liver transplant in women: Updated analysis of indications for liver transplant and ethnic and gender variances. Am. J. Gastroenterol. 2018, 113, 1649–1659. [Google Scholar] [CrossRef]
- Global Burden of Disease Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [Green Version]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e716. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.J.; Kim, W.; Kim, D.; Yoon, J.H.; Lee, K.; Kim, J.H.; Cho, E.J.; Lee, J.H.; Kim, H.Y.; Kim, Y.J.; et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e2159. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Rafiq, N.; Makhlouf, H.; Agrawal, R.; Kaur, I.; Younoszai, Z.; McCullough, A.; Goodman, Z.; Younossi, Z.M. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2013, 58, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Rafiq, N.; Younossi, Z.M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: A population-based study. Gut 2010, 59, 1410–1415. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Cerda-Reyes, E.; Higuera-de-la-Tijera, F.; Salas-Garcia, A.K.; Cabrera-Palma, S.; Cabrera-Alvarez, G.; Cortez-Hernandez, C.; Perez-Arredondo, L.A.; Puron-Gonzalez, E.; Coronado-Alejandro, E.; et al. Dyslipidemia as a risk factor for liver fibrosis progression in a multicentric population with non-alcoholic steatohepatitis. F1000Res 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, H.; Craig, D.; Barker, R.; Spiers, G.; Stow, D.; Anstee, Q.M.; Hanratty, B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020, 17, e1003100. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Loomis, A.K.; Fairburn-Beech, J.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018, 16, 130. [Google Scholar] [CrossRef]
- Loomba, R.; Wong, R.; Fraysse, J.; Shreay, S.; Li, S.; Harrison, S.; Gordon, S.C. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: A real world analysis of Medicare data. Aliment. Pharm. 2020, 51, 1149–1159. [Google Scholar] [CrossRef]
- Nielsen, E.M.; Anderson, K.P.; Marsden, J.; Zhang, J.; Schreiner, A.D. Nonalcoholic fatty liver disease underdiagnosis in primary care: What are we missing? J. Gen. Intern. Med. 2022, 37, 2587–2590. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Wong, V.W.; Chan, W.K.; Chitturi, S.; Chawla, Y.; Dan, Y.Y.; Duseja, A.; Fan, J.; Goh, K.L.; Hamaguchi, M.; Hashimoto, E.; et al. Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-part 1: Definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018, 33, 70–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, P.; Symonds, A.; Barritt, A.S., 4th. Therapy for nonalcoholic fatty liver disease: Current options and future directions. Clin. 2021, 43, 500–517. [Google Scholar] [CrossRef]
- Kanwal, F.; Shubrook, J.H.; Adams, L.A.; Pfotenhauer, K.; Wong, V.W.; Wright, E.; Abdelmalek, M.F.; Harrison, S.A.; Loomba, R.; Mantzoros, C.S.; et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021, 161, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Villota-Rivas, M.; Palayew, A.; Carrieri, P.; Colombo, M.; Ekstedt, M.; Esmat, G.; George, J.; Marchesini, G.; et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J. Hepatol. 2021, 76, 771–780. [Google Scholar] [CrossRef]
- Gracen, L.; Hayward, K.L.; Aikebuse, M.; Williams, S.; Russell, A.; O’Beirne, J.; Powell, E.E.; Valery, P.C. An exploration of barriers and facilitators to implementing a nonalcoholic fatty liver disease pathway for people with type 2 diabetes in primary care. Diabet. Med. 2022, 39, e14799. [Google Scholar] [CrossRef]
- Said, A.; Gagovic, V.; Malecki, K.; Givens, M.L.; Nieto, F.J. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann. Hepatol. 2013, 12, 758–765. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Makhlouf, H.R. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children. Clin. Liver Dis. 2016, 20, 293–312. [Google Scholar] [CrossRef] [Green Version]
- Monelli, F.; Venturelli, F.; Bonilauri, L.; Manicardi, E.; Manicardi, V.; Rossi, P.; Massari, M.; Ligabue, G.; Riva, N.; Schianchi, S.; et al. Systematic review of existing guidelines for NAFLD assessment. Hepatoma Res. 2021, 7, 25. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McGuire, D.K.; Gill, J.M.R. High circulating triglycerides are most commonly a marker of ectopic fat accumulation: Connecting the clues to advance lifestyle interventions. Circulation 2022, 146, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397.e310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treeprasertsuk, S.; Björnsson, E.; Enders, F.; Suwanwalaikorn, S.; Lindor, K.D. NAFLD fibrosis score: A prognostic predictor for mortality and liver complications among NAFLD patients. World J. Gastroenterol. 2013, 19, 1219–1229. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Newsome, P.N. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol. Hepatol. 2018, 3, 509–517. [Google Scholar] [CrossRef]
- Sattar, N.; Forrest, E.; Preiss, D. Non-alcoholic fatty liver disease. BMJ 2014, 349, g4596. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Houlihan, D.D.; Bentham, L.; Shaw, J.C.; Cramb, R.; Olliff, S.; Gill, P.S.; Neuberger, J.M.; Lilford, R.J.; Newsome, P.N. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J. Hepatol. 2012, 56, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Skelly, M.M.; James, P.D.; Ryder, S.D. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J. Hepatol. 2001, 35, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Cramb, R.; Davison, S.M.; Dillon, J.F.; Foulerton, M.; Godfrey, E.M.; Hall, R.; Harrower, U.; Hudson, M.; Langford, A.; et al. Guidelines on the management of abnormal liver blood tests. Gut 2018, 67, 6–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG clinical guideline: Evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Tapper, E.B.; Sengupta, N.; Lai, M.; Horowitz, G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disaese evaluation. Am. J. Med. 2016, 129, 115.e17–115.e22. [Google Scholar] [CrossRef]
- Tapper, E.B.; Saini, S.D.; Sengupta, N. Extensive testing or focused testing of patients with elevated liver enzymes. J. Hepatol. 2017, 66, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, S.; Zhang, J.; Dong, M.; Wang, Y.; Wang, M.; Xin, Y. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 10. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.P.; Desai, A.P.; Bajpai, S.; King, L.Y.; Sahani, D.V.; Corey, K.E. Gaps in recognition and evaluation of incidentally identified hepatic steatosis. Dig. Dis. Sci. 2015, 60, 333–338. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence. Non-Alcoholic Fatty Liver Disease (NAFLD): Assessment and Management. Available online: https://www.nice.org.uk/guidance/ng49/chapter/Recommendations (accessed on 3 April 2023).
- American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45, S46–S59. [Google Scholar] [CrossRef]
- Atsawarungruangkit, A.; Laoveeravat, P.; Promrat, K. Machine learning models for predicting non-alcoholic fatty liver disease in the general United States population: NHANES database. World J. Hepatol. 2021, 13, 1417–1427. [Google Scholar] [CrossRef]
- Noureddin, M.; Ntanios, F.; Malhotra, D.; Hoover, K.; Emir, B.; McLeod, E.; Alkhouri, N. Predicting NAFLD prevalence in the United States using National Health and Nutrition Examination Survey 2017–2018 transient elastography data and application of machine learning. Hepatol. Commun. 2022, 6, 1537–1548. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Non-Alcoholic Fatty Liver Disease: Assessment and Management. Available online: https://www.nice.org.uk/guidance/ng49/evidence/full-guideline-pdf-2548213310 (accessed on 3 April 2023).
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Ortiz-Lopez, C.; Lomonaco, R.; Orsak, B.; Freckleton, M.; Chintapalli, K.; Hardies, J.; Lai, S.; Solano, F.; Tio, F.; et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015, 35, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.K.; Johnson, L.A.; Germin, B.I.; Marcos, A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002, 8, 1114–1122. [Google Scholar] [CrossRef]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.; Gavish, E.; Gottlieb, P.; Katsnelson, L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am. J. Roentgenol. 2007, 189, W320–W323. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.-D.; Shi, J.-P.; Mi, Y.-Q.; Chen, G.-F.; Hu, X.; Liu, Y.-G.; Wang, X.-Y.; Pan, Q.; Chen, G.-Y.; et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. 2015, 35, 2392–2400. [Google Scholar] [CrossRef] [Green Version]
- Ratchatasettakul, K.; Rattanasiri, S.; Promson, K.; Sringam, P.; Sobhonslidsuk, A. The inverse effect of meal intake on controlled attenuation parameter and liver stiffness as assessed by transient elastography. BMC Gastroenterol. 2017, 17, 50. [Google Scholar] [CrossRef] [Green Version]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, e462–e468. [Google Scholar] [CrossRef] [Green Version]
- Noureddin, M.; Lam, J.; Peterson, M.R.; Middleton, M.; Hamilton, G.; Le, T.A.; Bettencourt, R.; Changchien, C.; Brenner, D.A.; Sirlin, C.; et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013, 58, 1930–1940. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Li, M.; Hamilton, G.; Zhang, Y.N.; Song, B. Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: A meta-analysis. Eur. Radiol. 2019, 29, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Castera, L. Non-invasive diagnosis of hepatic steatosis. Hepatol. Int. 2017, 11, 70–78. [Google Scholar] [CrossRef]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Sabag, A.; Barr, L.; Armour, M.; Armstrong, A.; Baker, C.J.; Twigg, S.M.; Chang, D.; Hackett, D.A.; Keating, S.E.; George, J.; et al. The effect of high-intensity interval training versus moderate-intensity continuous training on liver fat: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 862–881. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.A.; Gariani, K.; Oldani, G.; Delaune, V.; Morel, P.; Toso, C. Exercise-based interventions for nonalcoholic fatty liver disease: A meta-analysis and meta-regression. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 1398–1411. [Google Scholar] [CrossRef] [Green Version]
- Golabi, P.; Locklear, C.T.; Austin, P.; Afdhal, S.; Byrns, M.; Gerber, L.; Younossi, Z.M. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World J. Gastroenterol. 2016, 22, 6318–6327. [Google Scholar] [CrossRef]
- Babu, A.F.; Csader, S.; Lok, J.; Gómez-Gallego, C.; Hanhineva, K.; El-Nezami, H.; Schwab, U. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: A systematic review and meta-analysis. Nutrients 2021, 13, 3135. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef]
- Baker, C.J.; Martinez-Huenchullan, S.F.; D’Souza, M.; Xu, Y.; Li, M.; Bi, Y.; Johnson, N.A.; Twigg, S.M. Effect of exercise on hepatic steatosis: Are benefits seen without dietary intervention? A systematic review and meta-analysis. J. Diabetes 2021, 13, 63–77. [Google Scholar] [CrossRef]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol. Res. 2021, 51, 1013–1025. [Google Scholar] [CrossRef]
- Seagle, H.M.; Strain, G.W.; Makris, A.; Reeves, R.S.; American Dietetic Association. Position of the American Dietetic Association: Weight management. J. Am. Diet. Assoc. 2009, 109, 330–346. [Google Scholar] [CrossRef]
- Garcêz, L.S.; Avelar, C.R.; Fonseca, N.S.S.; Costa, P.R.F.; Lyra, A.C.; Cunha, C.M.; Jesus, R.P.; Oliveira, L.P.M. Effect of dietary carbohydrate and lipid modification on clinical and anthropometric parameters in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 1321–1337. [Google Scholar] [CrossRef]
- Houttu, V.; Csader, S.; Nieuwdorp, M.; Holleboom, A.G.; Schwab, U. Dietary interventions in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Nutr. 2021, 8, 716783. [Google Scholar] [CrossRef]
- Reddy, A.J.; George, E.S.; Roberts, S.K.; Tierney, A.C. Effect of dietary intervention, with or without co-interventions, on inflammatory markers in patients with nonalcoholic fatty liver disease: A systematic literature review. Nutr. Rev. 2019, 77, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef]
- Alon, L.; Corica, B.; Raparelli, V.; Cangemi, R.; Basili, S.; Proietti, M.; Romiti, G.F. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 33386. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, K.; Thoma, C.; Hollingsworth, K.G.; Cassidy, S.; Anstee, Q.M.; Day, C.P.; Trenell, M.I. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Sci. 2015, 129, 1097–1105. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [Green Version]
- GIHep. Fibrosis 4 Score. Available online: http://gihep.com/calculators/hepatology/fibrosis-4-score/ (accessed on 3 April 2023).
- Halfon, P.; Ansaldi, C.; Penaranda, G.; Chiche, L.; Dukan, P.; Stavris, C.; Plauzolles, A.; Retornaz, F.; Bourliere, M. Prospective screening of liver fibrosis in a primary care cohort using systematic calculation of fib-4 in routine results. PLoS ONE 2021, 16, e0254939. [Google Scholar] [CrossRef] [PubMed]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Mahady, S.E.; Macaskill, P.; Craig, J.C.; Wong, G.L.H.; Chu, W.C.W.; Chan, H.L.Y.; George, J.; Wong, V.W. Diagnostic accuracy of noninvasive fibrosis scores in a population of individuals with a low prevalence of fibrosis. Clin. Gastroenterol. Hepatol. 2017, 15, 1453–1460.E1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleg, N.; Issachar, A.; Sneh-Arbib, O.; Shlomai, A. AST to platelet ratio index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig. Liver Dis. 2017, 49, 1133–1138. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Tincopa, M.A.; Loomba, R. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Castéra, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1264–1281.E4. [Google Scholar] [CrossRef] [Green Version]
- Cox, B.; Trasolini, R.; Galts, C.; Yoshida, E.M.; Marquez, V. Comparing the performance of Fibrosis-4 and Non-Alcoholic Fatty Liver Disease Fibrosis Score with transient elastography scores of people with non-alcoholic fatty liver disease. Can. Liver J. 2021, 4, 275–282. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertot, L.C.; Jeffrey, G.P.; de Boer, B.; MacQuillan, G.; Garas, G.; Chin, J.; Huang, Y.; Adams, L.A. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018, 38, 1793–1802. [Google Scholar] [CrossRef]
- Petta, S.; Wong, V.W.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; Hiriart, J.B.; Lai-Hung Wong, G.; Vergniol, J.; Wing-Hung Chan, A.; Giannetti, A.; et al. Impact of obesity and alanine aminotransferase levels on the diagnostic accuracy for advanced liver fibrosis of noninvasive tools in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2019, 114, 916–928. [Google Scholar] [CrossRef]
- Joo, S.K.; Kim, W.; Kim, D.; Kim, J.H.; Oh, S.; Lee, K.L.; Chang, M.S.; Jung, Y.J.; So, Y.H.; Lee, M.S.; et al. Steatosis severity affects the diagnostic performances of noninvasive fibrosis tests in nonalcoholic fatty liver disease. Liver Int. 2018, 38, 331–341. [Google Scholar] [CrossRef]
- Siemens Healthineers. The Enhanced Liver Fibrosis (ELF) Blood Test: Literature Compendium Volume 1. Available online: https://www.siemens-healthineers.com/en-uk/laboratory-diagnostics/assays-by-diseases-conditions/liver-disease/elf-test (accessed on 3 April 2023).
- Xie, Q.; Zhou, X.; Huang, P.; Wei, J.; Wang, W.; Zheng, S. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: A meta-analysis. PLoS ONE 2014, 9, e92772. [Google Scholar] [CrossRef]
- Thiele, M.; Madsen, B.S.; Hansen, J.F.; Detlefsen, S.; Antonsen, S.; Krag, A. Accuracy of the enhanced liver fibrosis test vs. FibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 2018, 154, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Slavakis, A.; Koumerkeridis, G.; Katsinelos, P.; Kountouras, J. Noninvasive liver fibrosis tests in patients with nonalcoholic fatty liver disease: An external validation cohort. Horm. Metab. Res. 2019, 51, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Löffler, J.; Verheij, J.; Brosnan, M.J.; Böcskei, Z.; Anstee, Q.M.; Bossuyt, P.M.; et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence. FibroScan for Assessing Liver Fibrosis and Cirrhosis in Primary Care. Available online: https://www.nice.org.uk/advice/mib216/chapter/Expert-comments (accessed on 3 April 2023).
- Echosens. Fibroscan®. Available online: https://www.echosens.com/fibroscan/ (accessed on 3 April 2023).
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 156–163.e152. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Alavian, S.M.; Gholami-Fesharaki, M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Casp. J. Intern. Med. 2016, 7, 242–252. [Google Scholar]
- Castéra, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef]
- Echosens. New FibroScan® GO. Available online: https://www.flipsnack.com/98A8B8AA9F7/fibroscan-go_brochure-uk_web.html (accessed on 3 April 2023).
- Congly, S.E.; Shaheen, A.A.; Swain, M.G. Modelling the cost effectiveness of non-alcoholic fatty liver disease risk stratification strategies in the community setting. PLoS ONE 2021, 16, e0251741. [Google Scholar] [CrossRef]
- Salomone, F.; Micek, A.; Godos, J. Simple scores of fibrosis and mortality in patients with NAFLD: A systematic review with meta-analysis. J. Clin. Med. 2018, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Cui, H.; Li, N.; Wei, Y.; Lai, S.; Yang, Y.; Yin, X.; Chen, D.F. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol. Res. 2016, 46, 862–870. [Google Scholar] [CrossRef]
- Ismaiel, A.; Leucuta, D.C.; Popa, S.L.; Fagoonee, S.; Pellicano, R.; Abenavoli, L.; Dumitrascu, D.L. Noninvasive biomarkers in predicting nonalcoholic steatohepatitis and assessing liver fibrosis: Systematic review and meta-analysis. Panminerva Med. 2021, 63, 508–518. [Google Scholar] [CrossRef]


| Model | Risk/Prediction of Disease/Disease Stage | Predictors |
|---|---|---|
| ASCVD Risk Estimator | 10-year ASCVD risk intended for patients with LDL-cholesterol < 190 mg/dL (4.92 mmol/L), without ASCVD | Current age (years) |
| Sex (male/female) | ||
| Race (White/African American/other) | ||
| Systolic blood pressure (mmHg) | ||
| Diastolic blood pressure (mmHg) | ||
| Total cholesterol (mg/dL) | ||
| HDL-cholesterol (mg/dL) | ||
| LDL-cholesterol (mg/dL) | ||
| History of diabetes (yes/no) | ||
| Smoker (current/former/never) | ||
| On hypertension treatment (yes/no) | ||
| On a statin (yes/no) | ||
| On aspirin therapy (yes/no) | ||
| HeartScore® | 10-year risk of first-onset cardiovascular disease in European populations | Risk region (low risk/moderate risk/high risk/very high risk) |
| Age (years) | ||
| Sex (male/female) | ||
| Systolic blood pressure (mmHg) | ||
| Total cholesterol (mmol/L or mg/dL) | ||
| HDL-cholesterol (mmol/L) | ||
| Current smoker (yes/no) | ||
| Fibrosis-4 score | Prediction of liver fibrosis and cirrhosis | (Age (years) × AST (IU/L))/(Platelet count (109/L) × (square root (ALT (IU/L))) |
| NAFLD fibrosis score | Prediction of advanced fibrosis | −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (×109/L) − 0.66 × albumin (g/dL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, A.D.; Sattar, N. Identifying Patients with Nonalcoholic Fatty Liver Disease in Primary Care: How and for What Benefit? J. Clin. Med. 2023, 12, 4001. https://doi.org/10.3390/jcm12124001
Schreiner AD, Sattar N. Identifying Patients with Nonalcoholic Fatty Liver Disease in Primary Care: How and for What Benefit? Journal of Clinical Medicine. 2023; 12(12):4001. https://doi.org/10.3390/jcm12124001
Chicago/Turabian StyleSchreiner, Andrew D., and Naveed Sattar. 2023. "Identifying Patients with Nonalcoholic Fatty Liver Disease in Primary Care: How and for What Benefit?" Journal of Clinical Medicine 12, no. 12: 4001. https://doi.org/10.3390/jcm12124001