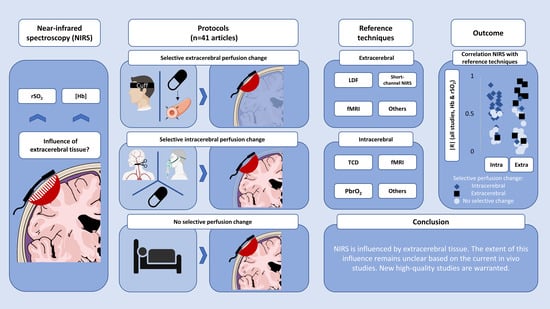
The Influence of Extracerebral Tissue on Continuous Wave Near-Infrared Spectroscopy in Adults: A Systematic Review of In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategy
2.3. Search Concepts
2.4. Inclusion Criteria
2.5. Critical Appraisal
2.6. Software and Statistical Analysis
3. Results
3.1. Literature Screening
3.2. General Characteristics
3.3. Study Methodology
3.4. Critical Appraisal
3.5. Comparison of Haemoglobin Concentrations and Reference Techniques
3.5.1. Extracerebral Correction Methods
3.5.2. Source–Detector Separation (SDS)
3.6. Comparison of Oxygen Saturation Indices and Reference Techniques
3.7. Studies without Within-Subject Comparisons with Reference Techniques
4. Discussion
4.1. Main Findings
4.2. Interpretation of the Results
4.3. The Problem of Quantifying Extracerebral Influence
4.4. Methodological Recommendations for Investigations of Extracerebral Influence
4.5. Implications for NIRS in Clinical Practice
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (FNIRS) Development and Fields of Application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Obrig, H.; Villringer, A. Beyond the Visible—Imaging the Human Brain with Light. J. Cereb. Blood Flow Metab. 2003, 23, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheeren, T.W.; Schober, P.; Schwarte, L.A. Monitoring Tissue Oxygenation by near Infrared Spectroscopy (NIRS): Background and Current Applications. J. Clin. Monit. Comput. 2012, 26, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional Near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Khan, J.M.; McInnis, C.L.; Ross-White, A.; Day, A.G.; Norman, P.A.; Boyd, J.G. Overview and Diagnostic Accuracy of Near Infrared Spectroscopy in Carotid Endarterectomy: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. Br. J. Anaesth. 2009, 103, i3–i13. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, H.L.; Ganzel, B.L.; Austin, E.H. Cerebral Oximetry for Cardiac and Vascular Surgery. Semin. Cardiothorac. Vasc. Anesth. 2004, 8, 147–166. [Google Scholar] [CrossRef]
- Haeussinger, F.B.; Heinzel, S.; Hahn, T.; Schecklmann, M.; Ehlis, A.-C.; Fallgatter, A.J. Simulation of Near-Infrared Light Absorption Considering Individual Head and Prefrontal Cortex Anatomy: Implications for Optical Neuroimaging. PLoS ONE 2011, 6, e26377. [Google Scholar] [CrossRef] [Green Version]
- Strangman, G.E.; Zhang, Q.; Li, Z. Scalp and Skull Influence on near Infrared Photon Propagation in the Colin27 Brain Template. Neuroimage 2014, 85 Pt 1, 136–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Q.; Liu, D.; Yang, C.; Sun, J. Influence of Extracerebral Layers on Estimates of Optical Properties with Continuous Wave Near Infrared Spectroscopy: Analysis Based on Multi-Layered Brain Tissue Architecture and Monte Carlo Simulation. Comput. Assist. Surg. 2019, 24, 144–150. [Google Scholar] [CrossRef]
- Afshari, A.; Ghassemi, P.; Lin, J.; Halprin, M.; Wang, J.; Mendoza, G.; Weininger, S.; Pfefer, T.J. Cerebral Oximetry Performance Testing with a 3D-Printed Vascular Array Phantom. Biomed. Opt. Express 2019, 10, 3731–3746. [Google Scholar] [CrossRef]
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. NeuroImage 2014, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-Infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies The PRISMA-DTA Statement. JAMA J. Am. Med. Assoc. 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Sharma, S.D.; Park, E.; Purcell, P.L.; Gordon, K.A.; Papsin, B.C.; Cushing, S.L. Age-Related Variability in Pediatric Scalp Thickness: Implications for Auditory Prostheses. Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109853. [Google Scholar] [CrossRef]
- Smith, K.; Politte, D.; Reiker, G.; Nolan, T.S.; Hildebolt, C.; Mattson, C.; Tucker, D.; Prior, F.; Turovets, S.; Larson-Prior, L.J. Automated Measurement of Pediatric Cranial Bone Thickness and Density from Clinical Computed Tomography. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 4462–4465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; McIntosh, E.J.; Unger, S.; Haddaway, N.R.; Kecke, S.; Schiemann, J.; Wilhelm, R. Online Tools Supporting the Conduct and Reporting of Systematic Reviews and Systematic Maps: A Case Study on CADIMA and Review of Existing Tools. Environ. Evid. 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- de Quirós, M.B.; Douma, E.H.; van den Akker-Scheek, I.; Lamoth, C.J.C.; Maurits, N.M. Quantification of Movement in Stroke Patients under Free Living Conditions Using Wearable Sensors: A Systematic Review. Sensors 2022, 22, 1050. [Google Scholar] [CrossRef]
- Rohatgi, A. Webplotdigitizer, Version 4.6; Pacifica, CA, USA. September 2022. Available online: https://automeris.io/WebPlotDigitizer/citation.html (accessed on 4 April 2023).
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogoh, S.; Sato, K.; Okazaki, K.; Miyamoto, T.; Secher, F.; Sørensen, H.; Rasmussen, P.; Secher, N.H. A Decrease in Spatially Resolved Near-Infrared Spectroscopy-Determined Frontal Lobe Tissue Oxygenation by Phenylephrine Reflects Reduced Skin Blood Flow. Anesth. Analg. 2014, 118, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD Signal. Annu. Rev. Physiol. 2004, 66, 735–769. [Google Scholar] [CrossRef] [PubMed]
- Delpy, D.T.; Cope, M.; Van Der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of Optical Pathlength through Tissue from Direct Time of Flight Measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Liebeskind, D.S. Collateral Circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef] [Green Version]
- Totaro, R.; Barattelli, G.; Quaresima, V.; Carolei, A.; Ferrari, M. Evaluation of Potential Factors Affecting the Measurement of Cerebrovascular Reactivity by Near-Infrared Spectroscopy. Clin. Sci. 1998, 95, 497–504. [Google Scholar] [CrossRef]
- Yang, H.C.; Liang, Z.; Vike, N.L.; Lee, T.; Rispoli, J.V.; Nauman, E.A.; Talavage, T.M.; Tong, Y. Characterizing Near-Infrared Spectroscopy Signal under Hypercapnia. J. Biophotonics 2020, 13, e202000173. [Google Scholar] [CrossRef]
- Holzschuh, M.; Woertgen, C.; Metz, C.; Brawanski, A. Comparison of Changes in Cerebral Blood Flow and Cerebral Oxygen Saturation Measured by Near Infrared Spectroscopy (NIRS) after Acetazolamide. Acta Neurochir. (Wien) 1997, 139, 58–62. [Google Scholar] [CrossRef]
- Smielewski, P.; Czosnyka, M.; Pickard, J.D.; Kirkpatrick, P. Assessment of Cerebrovascular Reactivity in Patients with Carotid Artery Disease Using Near-Infrared Spectroscopy. Acta Neurochir. Suppl. 1998, 1998, 263–265. [Google Scholar] [CrossRef]
- Smielewski, P.; Kirkpatrick, P.; Minhas, P.; Pickard, J.D.; Czosnyka, M. Can Cerebrovascular Reactivity Be Measured with Near-Infrared Spectroscopy? Stroke 1995, 26, 2285–2292. [Google Scholar] [CrossRef]
- Kirkpatrick, P.J.; Lam, J.; Al-Rawi, P.; Smielewski, P.; Czosnyka, M. Defining Thresholds for Critical Ischemia by Using Near-Infrared Spectroscopy in the Adult Brain. J. Neurosurg. 1998, 89, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Yanagisawa, S.; Tanaka, N.; Funane, T.; Kiguchi, M.; Sørensen, H.; Secher, N.H.; Ogoh, S. Influence of Skin Blood Flow and Source-Detector Distance on near-Infrared Spectroscopy-Determined Cerebral Oxygenation in Humans. Clin. Physiol. Funct. Imaging 2015, 35, 237–244. [Google Scholar] [CrossRef]
- Schecklmann, M.; Mann, A.; Langguth, B.; Ehlis, A.C.; Fallgatter, A.J.; Haeussinger, F.B. The Temporal Muscle of the Head Can Cause Artifacts in Optical Imaging Studies with Functional Near-Infrared Spectroscopy. Front. Hum. Neurosci. 2017, 11, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Takikawa, Y.; Kawagoe, R.; Shibuya, S.; Iwano, T.; Kitazawa, S. Influence of Skin Blood Flow on Near-Infrared Spectroscopy Signals Measured on the Forehead during a Verbal Fluency Task. Neuroimage 2011, 57, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Funane, T.; Sato, H.; Yahata, N.; Takizawa, R.; Nishimura, Y.; Kinoshita, A.; Katura, T.; Atsumori, H.; Fukuda, M.; Kasai, K.; et al. Concurrent FNIRS-FMRI Measurement to Validate a Method for Separating Deep and Shallow FNIRS Signals by Using Multidistance Optodes. Neurophotonics 2015, 2, 015003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeussinger, F.B.; Dresler, T.; Heinzel, S.; Schecklmann, M.; Fallgatter, A.J.; Ehlis, A.C. Reconstructing Functional Near-Infrared Spectroscopy (FNIRS) Signals Impaired by Extra-Cranial Confounds: An Easy-to-Use Filter Method. Neuroimage 2014, 95, 69–79. [Google Scholar] [CrossRef]
- Sato, H.; Yahata, N.; Funane, T.; Takizawa, R.; Katura, T.; Atsumori, H.; Nishimura, Y.; Kinoshita, A.; Kiguchi, M.; Koizumi, H.; et al. A NIRS-FMRI Investigation of Prefrontal Cortex Activity during a Working Memory Task. Neuroimage 2013, 83, 158–173. [Google Scholar] [CrossRef]
- Heinzel, S.; Haeussinger, F.B.; Hahn, T.; Ehlis, A.C.; Plichta, M.M.; Fallgatter, A.J. Variability of (Functional) Hemodynamics as Measured with Simultaneous FNIRS and FMRI during Intertemporal Choice. Neuroimage 2013, 71, 125–134. [Google Scholar] [CrossRef]
- Canova, D.; Roatta, S.; Bosone, D.; Micieli, G. Inconsistent Detection of Changes in Cerebral Blood Volume by near Infrared Spectroscopy in Standard Clinical Tests. J. Appl. Physiol. 2011, 110, 1646–1655. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Tisdall, M.; Delpy, D.T.; Smith, M.; Elwell, C.E. Measurement of Cerebral Tissue Oxygenation in Young Healthy Volunteers during Acetazolamide Provocation: A Transcranial Doppler and near-Infrared Spectroscopy Investigation. Adv. Exp. Med. Biol. 2008, 614, 389–396. [Google Scholar] [CrossRef]
- Germon, T.J.; Young, A.E.R.; Manara, A.R.; Nelson, R.J. Extracerebral Absorption of near Infrared Light Influences the Detection of Increased Cerebral Oxygenation Monitored by near Infrared Spectroscopy. J. Neurol. Neurosurg. Psychiatry 1995, 58, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Picton, P.; Vlisides, P.E.; Teig, M.K.; Heth, J.A.; Orringer, D.; Brooks, J.; McKinney, A.; Mentz, G.; Mashour, G.A. Correlation between Brain Tissue Oxygen Tension and Regional Cerebral Oximetry in Uninjured Human Brain under Conditions of Changing Ventilation Strategy. J. Clin. Monit. Comput. 2022, 36, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, P.G.; Smielewski, P.; Kirkpatrick, P.J. Evaluation of a Near-Infrared Spectrometer (NIRO 300) for the Detection of Intracranial Oxygenation Changes in the Adult Head. Stroke 2001, 32, 2492–2499. [Google Scholar] [CrossRef]
- Yoshitani, K.; Kawaguchi, M.; Tatsumi, K.; Kitaguchi, K.; Furuya, H. A Comparison of the INVOS 4100 and the NIRO 300 Near-Infrared Spectrophotometers. Anesth. Analg. 2002, 94, 586–590. [Google Scholar] [CrossRef]
- Henson, L.C.; Temp, J.A.; Ward, D.S. Accuracy of a Cerebral Oximeter in Healthy Volunteers under Conditions of Isocapnic Hypoxia. Anesthesiology 1998, 88, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Grubhofer, G.; Tonninger, W.; Keznickl, P.; Skyllouriotis, P.; Ehrlich, M.; Hiesmayr, M.; Lassnigg, A. A Comparison of the Monitors INVOS 3100 and NIRO 500 in Detecting Changes in Cerebral Oxygenation. Acta Anaesthesiol. Scand. 1999, 43, 470–475. [Google Scholar] [CrossRef]
- Samra, S.K.; Stanley, J.C.; Zelenock, G.B.; Dorje, P. An Assessment of Contributions Made by Extracranial Tissues during Cerebral Oximetry. J. Neurosurg. Anesth. 1999, 11, 1–5. [Google Scholar] [CrossRef]
- Cho, H.; Nemoto, E.M.; Yonas, H.; Balzer, J.; Sclabassi, R.J. Cerebral Monitoring by Means of Oximetry and Somatosensory Evoked Potentials during Carotid Endarterectomy. J. Neurosurg. 1998, 89, 533–538. [Google Scholar] [CrossRef]
- Kato, S.; Yoshitani, K.; Kubota, Y.; Inatomi, Y.; Ohnishi, Y. Effect of Posture and Extracranial Contamination on Results of Cerebral Oximetry by Near-Infrared Spectroscopy. J. Anesth. 2017, 31, 103–110. [Google Scholar] [CrossRef]
- Germon, T.J.; Kane, N.M.; Manara, A.R.; Nelson, R.J. Near-Infrared Spectroscopy in Adults: Effects of Extracranial Ischaemia and Intracranial Hypoxia on Estimation of Cerebral Oxygenation. Br. J. Anaesth. 1994, 73, 503–506. [Google Scholar] [CrossRef]
- Greenberg, S.; Murphy, G.; Shear, T.; Patel, A.; Simpson, A.; Szokol, J.; Avram, M.J.; Vender, J. Extracranial Contamination in the INVOS 5100C versus the FORE-SIGHT ELITE Cerebral Oximeter: A Prospective Observational Crossover Study in Volunteers. Can. J. Anesth. 2016, 63, 24–30, Erratum in Can. J. Anesth. 2016, 63, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davie, S.N.; Grocott, H.P. Impact of Extracranial Contamination on Regional Cerebral Oxygen Saturation: A Comparison of Three Cerebral Oximetry Technologies. Anesthesiology 2012, 116, 834–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, H.; Rasmussen, P.; Siebenmann, C.; Zaar, M.; Hvidtfeldt, M.; Ogoh, S.; Sato, K.; Kohl-Bareis, M.; Secher, N.H.; Lundby, C. Extra-Cerebral Oxygenation Influence on near-Infrared-Spectroscopy-Determined Frontal Lobe Oxygenation in Healthy Volunteers: A Comparison between INVOS-4100 and NIRO-200NX. Clin. Physiol. Funct. Imaging 2015, 35, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Germon, T.J.; Evans, P.D.; Barnett, N.J.; Wall, P.; Manara, A.R.; Nelson, R.J. Cerebral near Infrared Spectroscopy: Emitter-Detector Separation Must Be Increased. Br. J. Anaesth. 1999, 82, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Germon, T.J.; Evans, P.D.; Manara, A.R.; Barnett, N.J.; Wall, P.; Nelson, R.J. Sensitivity of near Infrared Spectroscopy to Cerebral and Extra-Cerebral Oxygenation Changes Is Determined Dy Emitter-Detector Separation. J. Clin. Monit. Comput. 1998, 14, 353–360. [Google Scholar] [CrossRef]
- Tateishi, A.; Maekawa, T.; Soejima, Y.; Sadamitsu, D.; Yamamoto, M.; Matsushita, M.; Nakashima, K. Qualitative Comparison of Carbon Dioxide-Induced Change in Cerebral near-Infrared Spectroscopy versus Jugular Venous Oxygen Saturation in Adults with Acute Brain Disease. Crit. Care Med. 1995, 23, 1734–1738. [Google Scholar] [CrossRef]
- Hirasawa, A.; Kaneko, T.; Tanaka, N.; Funane, T.; Kiguchi, M.; Sørensen, H.; Secher, N.H.; Ogoh, S. Near-Infrared Spectroscopy Determined Cerebral Oxygenation with Eliminated Skin Blood Flow in Young Males. J. Clin. Monit. Comput. 2016, 30, 243–250. [Google Scholar] [CrossRef]
- Moerman, A.T.; Vandenheuvel, M.; Tuybens, P.J.; Van Gompel, C.; De Hert, S.G. Incongruous Effect of Phenylephrine on Changes in Cerebral Blood Volume Measured by Near-Infrared Spectroscopy (NIRS) Indicating Extracranial Contamination. J. Clin. Monit. Comput. 2021, 36, 745–750. [Google Scholar] [CrossRef]
- Moerman, A.; Wouters, P. Near-Infrared Spectroscopy (NIRS) Monitoring in Contemporary Anesthesia and Critical Care. Acta Anaesthesiol. Belg 2010, 61, 185–194. [Google Scholar]
- Yao, F.S.F.; Tseng, C.C.A.; Ho, C.Y.A.; Levin, S.K.; Illner, P. Cerebral Oxygen Desaturation Is Associated with Early Postoperative Neuropsychological Dysfunction in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 552–558. [Google Scholar] [CrossRef]
- Fischer, G.W.; Lin, H.M.; Krol, M.; Galati, M.F.; Luozzo, G.; Griepp, R.B.; Reich, D.L. Noninvasive Cerebral Oxygenation May Predict Outcome in Patients Undergoing Aortic Arch Surgery. J. Thorac. Cardiovasc. Surg. 2011, 141, 815–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, S.; Sutter, F.; Ferdinand, F.; Trace, C. Optimizing Intraoperative Cerebral Oxygen Delivery Using Noninvasive Cerebral Oximetry Decreases the Incidence of Stroke for Cardiac Surgical Patients. Heart Surg. Forum 2004, 7, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Orihashi, K.; Sueda, T.; Okada, K.; Imai, K. Near-Infrared Spectroscopy for Monitoring Cerebral Ischemia during Selective Cerebral Perfusion. Eur. J. Cardio-Thorac. Surg. 2004, 26, 907–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, C.; Thelin, S. Regional Cerebral Saturation Monitoring with Near-Infrared Spectroscopy during Selective Antegrade Cerebral Perfusion: Diagnostic Performance and Relationship to Postoperative Stroke. J. Thorac. Cardiovasc. Surg. 2006, 131, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Higami, T.; Kozawa, S.; Asada, T.; Obo, H.; Gan, K.; Iwahashi, K.; Nohara, H. Retrograde Cerebral Perfusion versus Selective Cerebral Perfusion as Evaluated by Cerebral Oxygen Saturation during Aortic Arch Reconstruction. Ann. Thorac. Surg. 1999, 67, 1091–1096. [Google Scholar] [CrossRef]
- Lam, S.; Liu, H.; Jian, Z.; Settels, J.; Bohringer, C. Intraoperative Invasive Blood Pressure Monitoring and the Potential Pitfalls of Invasively Measured Systolic Blood Pressure. Cureus 2021, 13, e17610. [Google Scholar] [CrossRef]
- Lange, F.; Tachtsidis, I. Clinical Brain Monitoring with Time Domain NIRS: A Review and Future Perspectives. Appl. Sci. 2019, 9, 1612. [Google Scholar] [CrossRef] [Green Version]
- Bein, B.; Cavus, E.; Stadlbauer, K.H.; Tonner, P.H.; Steinfath, M.; Scholz, J.; Dörges, V. Monitoring of Cerebral Oxygenation with near Infrared Spectroscopy and Tissue Oxygen Partial Pressure during Cardiopulmonary Resuscitation in Pigs. Eur. J. Anaesthesiol. 2006, 23, 501–509. [Google Scholar] [CrossRef]
- Nosrati, R.; Lin, S.; Ramadeen, A.; Monjazebi, D.; Dorian, P.; Toronov, V. Cerebral Hemodynamics and Metabolism during Cardiac Arrest and Cardiopulmonary Resuscitation Using Hyperspectral near Infrared Spectroscopy. Circ. J. 2017, 81, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Dixon, B.; Turner, R.; Christou, C. Assessment of a Non-Invasive Brain Oximeter in a Sheep Model of Acute Brain Injury. Med. Devices Evid. Res. 2019, 12, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.T.; Diop, M.; Tichauer, K.M.; Lee, Y.T.; St. Lawrence, K. Measurement of Cerebral Blood Flow in the Adult Pig by Depth-Resolved Broadband near-Infrared Spectroscopy. In Proceedings of the Journal of Cerebral Blood Flow & Metabolism; Elliott, J.T., Ed.; Imaging Division, Lawson Health Research Institute: London, ON, Canada, 2009; Volume 29, pp. S394–S406. [Google Scholar]
- Nosrati, R.; Ramadeen, A.; Hu, X.; Woldemichael, E.; Kim, S.; Dorian, P.; Toronov, V. Simultaneous Measurement of Cerebral and Muscle Tissue Parameters during Cardiac Arrest and Cardiopulmonary Resuscitation. In Proceedings of the Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics II; SPIE: San Francisco, CA, USA, 2015; Volume 9305, p. 93051G. [Google Scholar]
- Gerega, A.; Lachowska, M.; Morrison, L.; St. Lawrence, K.; Liebert, A. Multi-Wavelength Time-Resolved NIRS Measurements in Pigs During Inflow and Washout of ICG: Assessment of Extracerebral Signal Contamination. In Proceedings of the Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS); OSA: Washington, DC, USA, 2018; p. JTh3A.62. [Google Scholar]
- Lotfabadi, S.S.; Toronov, V.; Ramadeen, A.; Hu, X.; Kim, S.; Dorian, P.; Hare, G.M.T. Quantitative Assessment of Brain Tissue Oxygenation in Porcine Models of Cardiac Arrest and Cardiopulmonary Resuscitation Using Hyperspectral Near-Infrared Spectroscopy. In Proceedings of the Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics; SPIE: San Francisco, CA, USA, 2014; Volume 8928. [Google Scholar]
- Zarei, M.; Ansari, M.A.; Zare, K. The Temporal Confounding Effects of Extra-Cerebral Contamination Factors on the Hemodynamic Signal Measured by Functional near-Infrared Spectroscopy. J. Lasers Med. Sci. 2019, 10, S73–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smielewski, P.; Czosnyka, M.; Pickard, J.D.; Kirkpatrick, P. Clinical Evaluation of Near-Infrared Spectroscopy for Testing Cerebrovascular Reactivity in Patients with Carotid Artery Disease. Stroke 1997, 28, 331–338. [Google Scholar] [CrossRef]
- Takeda, N.; Fujita, K.; Katayama, S.; Tamaki, N. Cerebral Oximetry for the Detection of Cerebral Ischemia during Temporary Carotid Artery Occlusion. Neurol. Med. Chir. 2000, 40, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrink, J.M.; Kempf, F.; Schreiber, S.; Uludag, K.; Kohl, M.; Valdueza, J.M.; Villringer, A.; Obrig, H. Functional Brain Imaging by CW-NIRS Coregistered by Blood Flow Monitors. In Proceedings of the Photon Migration and Diffuse-Light Imaging; SPIE: San Francisco, CA, USA, 2003; Volume 5138. [Google Scholar]
- Toronov, V.Y.; Webb, A.; Choi, J.H.; Wolf, M.; Gratton, E.; Hueber, D.M. Simultaneous Functional Magnetic Resonance and Near-Infrared Imaging of Adult Human Brain. In Proceedings of the Optical Tomography and Spectroscopy of Tissue IV; SPIE: San Francisco, CA, USA, 2001; Volume 4250, pp. 380–382. [Google Scholar]
- Duncan, L.A.; Ruckley, C.V.; Wildsmith, J.A. Cerebral Oximetry: A Useful Monitor during Carotid Artery Surgery. Anaesthesia 1995, 50, 1041–1045. [Google Scholar] [CrossRef]
- Lam, J.M.K.K.; Smielewski, P.; Al-Rawi, P.; Griffiths, P.; Pickard, J.D.; Kirkpatrick, P.J. Internal and External Carotid Contributions to Near-Infrared Spectroscopy during Carotid Endarterectomy. Stroke 1997, 28, 906–911. [Google Scholar] [CrossRef] [PubMed]




| Domain | Screening Criteria | ||
|---|---|---|---|
| Population | Human adults (>18 years) or adult large animal models | ||
| Outcome measure | Cerebral continuous wave or frequency-domain near-infrared spectroscopy: OxyHb, HHb, Total Hb (tHb), Hbdiff (difference between OxyHb and HHb), or a tissue oxygen saturation index (rSO2). Other manufacturer-specific oxygenation indices are allowed, e.g., TOI, TSI, StO2. | ||
| Exposure | Influence of extracerebral tissue (scalp, skull, cerebrospinal fluid, or dura mater) was an explicit goal of the research, i.e., it is a primary or secondary research question, or primary or secondary research goal | AND | Reference techniques for both the intra- and extracerebral tissue that measure blood flow, Hb concentrations or tissue oxygen saturation |
| OR | |||
| A perfusion modification protocol selective for the intra- or extracerebral tissue | |||
| Study quality and type | Empirical peer-reviewed research presenting original data. No reviews, book chapters, commentaries, conference abstracts without a full text, or case reports | ||
| Study methodology | Only in vivo studies; no computational modelling, phantom, or tissue sample studies | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleveld, N.; Esquivel-Franco, D.C.; Drost, G.; Absalom, A.R.; Zeebregts, C.J.; de Vries, J.-P.P.M.; Elting, J.W.J.; Maurits, N.M. The Influence of Extracerebral Tissue on Continuous Wave Near-Infrared Spectroscopy in Adults: A Systematic Review of In Vivo Studies. J. Clin. Med. 2023, 12, 2776. https://doi.org/10.3390/jcm12082776
Eleveld N, Esquivel-Franco DC, Drost G, Absalom AR, Zeebregts CJ, de Vries J-PPM, Elting JWJ, Maurits NM. The Influence of Extracerebral Tissue on Continuous Wave Near-Infrared Spectroscopy in Adults: A Systematic Review of In Vivo Studies. Journal of Clinical Medicine. 2023; 12(8):2776. https://doi.org/10.3390/jcm12082776
Chicago/Turabian StyleEleveld, Nick, Diana C. Esquivel-Franco, Gea Drost, Anthony R. Absalom, Clark J. Zeebregts, Jean-Paul P. M. de Vries, Jan Willem J. Elting, and Natasha M. Maurits. 2023. "The Influence of Extracerebral Tissue on Continuous Wave Near-Infrared Spectroscopy in Adults: A Systematic Review of In Vivo Studies" Journal of Clinical Medicine 12, no. 8: 2776. https://doi.org/10.3390/jcm12082776