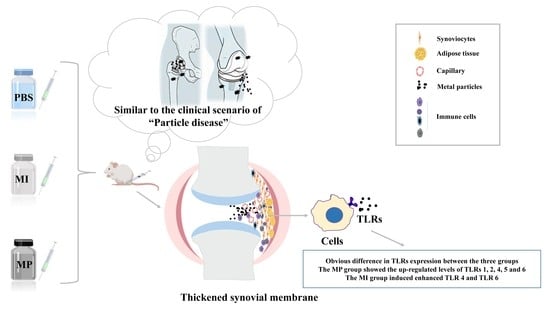
The Expression Levels of Toll-like Receptors after Metallic Particle and Ion Exposition in the Synovium of a Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Co28Cr6Mo Particles/Ions Generation
2.2. Removal of Endotoxins
2.3. Animals and Intraarticular Injection
2.4. Immunohistochemistry
2.5. Ethics
2.6. Statistics
3. Results
3.1. Expression of TLR 1
3.2. Expression of TLR 2
3.3. Expression of TLR 4
3.4. Expression of TLR 5
3.5. Expression of TLR 6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonitz-Heincke, A.; Tillmann, J.; Klinder, A.; Krueger, S.; Kretzer, J.P.; Høl, P.J.; Paulus, A.C.; Bader, R. The Impact of Metal Ion Exposure on the Cellular Behavior of Human Osteoblasts and PBMCs: In Vitro Analyses of Osteolytic Processes. Materials 2017, 10, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, S.B.; Gallo, J.; Gibon, E.F.; Takagi, M. Diagnosis and management of implant debris-associated inflammation. Expert Rev. Med. Devices 2019, 17, 41–56. [Google Scholar] [CrossRef]
- Price, A.J.; Alvand, A.; Troelsen, A.; Katz, J.N.; Hooper, G.; Gray, A.; Carr, A.; Beard, D. Knee replacement. Lancet 2018, 392, 1672–1682. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Sansone, V. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised histopathological consensus classification of joint implant related pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, G.; Pandit, H.; Kliskey, K.; Murray, D.; Gill, H.; Athanasou, N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009, 80, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.B.; Gibon, E.; Pajarinen, J.; Lin, T.; Keeney, M.; Ren, P.-G.; Nich, C.; Yao, Z.; Egashira, K.; Yang, F.; et al. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J. R. Soc. Interface 2014, 11, 20130962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzschneider, S.; Lorber, V.; Dedic, M.; Paulus, A.C.; Schröder, C.; Gottschalk, O.; Schmitt-Sody, M.; Jansson, V. Biological activity and migration of wear particles in the knee joint: An in vivo comparison of six different polyethylene materials. J. Mater. Sci. Mater. Electron. 2014, 25, 1599–1612. [Google Scholar] [CrossRef]
- Gibon, E.; Goodman, S.B. The Biologic Response to Bearing Materials. Orthop. Knowl. Online J. 2016, 14, 29104715. [Google Scholar]
- Takagi, M.; Takakubo, Y.; Pajarinen, J.; Naganuma, Y.; Oki, H.; Maruyama, M.; Goodman, S.B. Danger of frustrated sensors: Role of Toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements. J. Orthop. Transl. 2017, 10, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Keegan, G.M.; Learmonth, I.D.; Case, C.P. Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Savarino, L.M.; Ciapetti, G.; Baldini, N. Biological effects of metal degradation in hip arthroplasties. Crit. Rev. Toxicol. 2017, 48, 170–193. [Google Scholar] [CrossRef]
- Cobelli, N.; Scharf, B.; Crisi, G.M.; Hardin, J.; Santambrogio, L. Mediators of the inflammatory response to joint replacement devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lähdeoja, T.; Pajarinen, J.; Kouri, V.-P.; Sillat, T.; Salo, J.; Konttinen, Y.T. Toll-like receptors and aseptic loosening of hip endoprosthesis-a potential to respond against danger signals? J. Orthop. Res. 2009, 28, 184–190. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Amer, L.D.; Saleh, L.S.; Walker, C.; Thomas, S.; Janssen, W.J.; Alper, S.; Bryant, S.J. Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels. Acta Biomater. 2019, 100, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Tamaki, Y.; Hasegawa, H.; Takakubo, Y.; Konttinen, L.; Tiainen, V.-M.; Lappalainen, R.; Konttinen, Y.T.; Salo, J. Toll-like receptors in the interface membrane around loosening total hip replacement implants. J. Biomed. Mater. Res. Part A 2007, 81, 1017–1026. [Google Scholar] [CrossRef]
- Paulus, A.C.; Frenzel, J.; Ficklscherer, A.; Roßbach, B.P.; Melcher, C.; Jansson, V.; Utzschneider, S. Polyethylene wear particles induce TLR 2 upregulation in the synovial layer of mice. J. Mater. Sci. Mater. Med. 2013, 25, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Grandjean-Laquerriere, A.; Tabary, O.; Jacquot, J.; Richard, D.; Frayssinet, P.; Guenounou, M.; Laurent-Maquin, D.; Laquerriere, P.; Gangloff, S. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials 2007, 28, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Mackiewicz, Z.; Pöllänen, R.; Takagi, M.; Epstein, N.J.; Ma, T.; Goodman, S.B.; Konttinen, Y.T. Titanium particles modulate expression of Toll-like receptor proteins. J. Biomed. Mater. Res. Part A 2009, 92, 1528–1537. [Google Scholar] [CrossRef]
- Valladares, R.D.; Nich, C.; Zwingenberger, S.; Li, C.; Swank, K.R.; Gibon, E.; Rao, A.J.; Yao, Z.; Goodman, S.B. Toll-like receptors-2 and 4 are overexpressed in an experimental model of particle-induced osteolysis. J. Biomed. Mater. Res. Part A 2013, 102, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef]
- Bonham, K.; Orzalli, M.H.; Hayashi, K.; Wolf, A.I.; Glanemann, C.; Weninger, W.; Iwasaki, A.; Knipe, D.M.; Kagan, J.C. A Promiscuous Lipid-Binding Protein Diversifies the Subcellular Sites of Toll-like Receptor Signal Transduction. Cell 2014, 156, 705–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantafilou, M.; Gamper, F.G.; Haston, R.M.; Mouratis, M.A.; Morath, S.; Hartung, T.; Triantafilou, K. Membrane Sorting of Toll-like Receptor (TLR)-2/6 and TLR2/1 Heterodimers at the Cell Surface Determines Heterotypic Associations with CD36 and Intracellular Targeting. J. Biol. Chem. 2006, 281, 31002–31011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Catelas, I.; Petit, A.; Vali, H.; Fragiskatos, C.; Meilleur, R.; Zukor, D.J.; Antoniou, J.; Huk, O.L. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials 2005, 26, 2441–2453. [Google Scholar] [CrossRef]
- Paulus, A.C.; Ebinger, K.; Cheng, X.; Haßelt, S.; Weber, P.; Kretzer, J.P.; Bader, R.; Utzschneider, S. Local Biological Reactions and Pseudotumor-Like Tissue Formation in relation to Metal Wear in a Murine In Vivo Model. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Samelko, L.; Landgraeber, S.; McAllister, K.; Jacobs, J.; Hallab, N.J. TLR4 (not TLR2) dominate cognate TLR activity associated with CoCrMo implant particles. J. Orthop. Res. 2017, 35, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Dirmeier, S.C.; Haßelt, S.; Baur-Melnyk, A.; Kretzer, J.P.; Bader, R.; Utzschneider, S.; Paulus, A.C. Biological Reactions to Metal Particles and Ions in the Synovial Layer of Mice. Materials 2020, 13, 1044. [Google Scholar] [CrossRef] [Green Version]
- Athanasou, N.A. The pathobiology and pathology of aseptic implant failure. Bone Jt. Res. 2016, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Wimmer, M.A.; Utzschneider, S. Polyethylene and metal wear particles: Characteristics and biological effects. Semin. Immunopathol. 2011, 33, 257–271. [Google Scholar] [CrossRef] [PubMed]
- De Smet, K.; De Haan, R.; Calistri, A.; Campbell, P.; Ebramzadeh, E.; Pattyn, C.; Gill, H. Metal Ion Measurement as a Diagnostic Tool to Identify Problems with Metal-on-Metal Hip Resurfacing. J. Bone Jt. Surg. Am. Vol. 2008, 90, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.; Buckley, C.D.; Cañete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial tissue research: A state-of-the-art review. Nat. Rev. Rheumatol. 2017, 13, 463–475. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [Green Version]
- Perino, G.; Sunitsch, S.; Huber, M.; Ramirez, D.; Gallo, J.; Vaculova, J.; Natu, S.; Kretzer, J.P.; Müller, S.; Thomas, P.; et al. Diagnostic guidelines for the histological particle algorithm in the periprosthetic neo-synovial tissue. BMC Clin. Pathol. 2018, 18, 7. [Google Scholar] [CrossRef]
- Krenn, V.; Perino, G.; Wienert, S.; Saberi, D.; Hügle, T.; Hopf, F.; Huber, M.; Krenn, V. Histopathologische Diagnostik von Gelenkendoprothesen-assoziierten Erkrankungen. Der Hautarzt 2016, 67, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Vaculova, J.; Goodman, S.B.; Konttinen, Y.T.; Thyssen, J.P. Contributions of human tissue analysis to understanding the mechanisms of loosening and osteolysis in total hip replacement. Acta Biomater. 2014, 10, 2354–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzschneider, S.; Becker, F.; Grupp, T.M.; Sievers, B.; Paulus, A.; Gottschalk, O.; Jansson, V. Inflammatory response against different carbon fiber-reinforced PEEK wear particles compared with UHMWPE in vivo. Acta Biomater. 2010, 6, 4296–4304. [Google Scholar] [CrossRef] [PubMed]
- Lorber, V.; Paulus, A.C.; Buschmann, A.; Schmitt, B.; Grupp, T.M.; Jansson, V.; Utzschneider, S. Elevated cytokine expression of different PEEK wear particles compared to UHMWPE in vivo. J. Mater. Sci. Mater. Electron. 2013, 25, 141–149. [Google Scholar] [CrossRef]
- Burkandt, A.; Katzer, A.; Thaler, K.; Von Baehr, V.; Friedrich, R.E.; Rüther, W.; Amling, M.; Zustin, J. Proliferation of the synovial lining cell layer in suggested metal hypersensitivity. In Vivo 2011, 25, 679–686. [Google Scholar]
- Gallo, J.; Goodman, S.; Konttinen, Y.; Wimmer, M.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [Green Version]
- Zysk, S.P.; Gebhard, H.H.; Pellengahr, C.; Refior, H.J.; Plitz, W.; Messmer, K.; Veihelmann, A. Inflammatory responses to wear particles in vivo: A novel model in the murine knee joint. Der Orthop. 2003, 32, 305–311. [Google Scholar] [CrossRef]
- Bannon, D.I.; Bao, W.; Turner, S.D.; McCain, W.C.; Dennis, W.E.; Wolfinger, R.; Perkins, E.E.; Abounader, R. Gene expression in mouse muscle over time after nickel pellet implantation. Metallomics 2020, 12, 528–538. [Google Scholar] [CrossRef]
- Raghavan, B.; Martin, S.F.; Esser, P.; Goebeler, M.; Schmidt, M. Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep. 2012, 13, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Raghavan, B.; Müller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef]
- Herrero-Beaumont, G.; Pérez-Baos, S.; Pernaute, O.S.; Roman-Blas, J.A.; Lamuedra, A.; Largo, R. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem. Pharmacol. 2019, 165, 24–32. [Google Scholar] [CrossRef]
- Back, D.L.; Young, D.A.; Shimmin, A.J. How Do Serum Cobalt and Chromium Levels Change after Metal-on-Metal Hip Resurfacing? Clin. Orthop. Relat. Res. 2005, 438, 177–181. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Black, J.; Galante, J.O. Metal Release in Patients Who Have Had a Primary Total Hip Arthroplasty. A Prospective, Controlled, Longitudinal Study. J. Bone Jt. Surg. Am. Vol. 1998, 80, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, H.; Li, Z.; Wang, C.; Xiao, F.; Gao, Y.; Zhang, X.; Wang, P.; Peng, J.; Cai, G.; et al. The inhibition of RANKL expression in fibroblasts attenuate CoCr particles induced aseptic prosthesis loosening via the MyD88-independent TLR signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 1115–1122. [Google Scholar] [CrossRef]
- Liao, Y.; Hoffman, E.; Wimmer, M.; Fischer, A.; Jacobs, J.; Marks, L. CoCrMo metal-on-metal hip replacements. Phys. Chem. Chem. Phys. 2012, 15, 746–756. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Bin Lee, S.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, C.; Reinders, J.; Zietz, C.; Utzschneider, S.; Bader, R.; Kretzer, J.P. Characterization of polyethylene wear particle: The impact of methodology. Acta Biomater. 2013, 9, 9485–9491. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, S.; Paulus, A.; Datz, J.-C.; Schroeder, C.; Sievers, B.; Wegener, B.; Jansson, V. Influence of design and bearing material on polyethylene wear particle generation in total knee replacement. Acta Biomater. 2009, 5, 2495–2502. [Google Scholar] [CrossRef]
- Paulus, A.C.; Haßelt, S.; Jansson, V.; Giurea, A.; Neuhaus, H.; Grupp, T.M.; Utzschneider, S. Histopathological Analysis of PEEK Wear Particle Effects on the Synovial Tissue of Patients. BioMed Res. Int. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Shukhnova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ruenraroengsak, P.; Novak, P.; Berhanu, D.; Thorley, A.J.; Valsami-Jones, E.; Gorelik, J.; Korchev, Y.; Tetley, T.D. Respiratory epithelial cytotoxicity and membrane damage (holes) caused by amine-modified nanoparticles. Nanotoxicology 2011, 6, 94–108. [Google Scholar] [CrossRef]
- Zhang, X.; Mosser, D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2007, 214, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, E.M.; Beidelschies, M.A.; Tatro, J.M.; Goldberg, V.M.; Hise, A. Bacterial Pathogen-associated Molecular Patterns Stimulate Biological Activity of Orthopaedic Wear Particles by Activating Cognate Toll-like Receptors. J. Biol. Chem. 2010, 285, 32378–32384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassem, A.; Henning, P.; Kindlund, B.; Lindholm, C.; Lerner, U.H. TLR5, a novel mediator of innate immunity-induced osteoclastogenesis and bone loss. FASEB J. 2015, 29, 4449–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zysk, S.P.; Gebhard, H.H.; Kalteis, T.; Schmitt-Sody, M.; Jansson, V.; Messmer, K.; Veihelmann, A. Particles of All Sizes Provoke Inflammatory Responses In Vivo. Clin. Orthop. Relat. Res. 2005, 433, 258–264. [Google Scholar] [CrossRef]





| Content In | Co | Cr | Mo | Ni |
|---|---|---|---|---|
| stock solution | 13.7 mg/L | 4.3 mg/L | 0.8 mg/L | 1.7 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Jansson, V.; Kretzer, J.P.; Bader, R.; Utzschneider, S.; Paulus, A.C. The Expression Levels of Toll-like Receptors after Metallic Particle and Ion Exposition in the Synovium of a Murine Model. J. Clin. Med. 2021, 10, 3489. https://doi.org/10.3390/jcm10163489
Cheng X, Jansson V, Kretzer JP, Bader R, Utzschneider S, Paulus AC. The Expression Levels of Toll-like Receptors after Metallic Particle and Ion Exposition in the Synovium of a Murine Model. Journal of Clinical Medicine. 2021; 10(16):3489. https://doi.org/10.3390/jcm10163489
Chicago/Turabian StyleCheng, Xiangyun, Volkmar Jansson, Jan Philippe Kretzer, Rainer Bader, Sandra Utzschneider, and Alexander C. Paulus. 2021. "The Expression Levels of Toll-like Receptors after Metallic Particle and Ion Exposition in the Synovium of a Murine Model" Journal of Clinical Medicine 10, no. 16: 3489. https://doi.org/10.3390/jcm10163489